From Mount Sinai
Polycystic Kidney Disease
If one or both of your parents has been diagnosed with or carries the gene for polycystic kidney disease (PKD), you have a chance of developing the disease. It is one of the most common genetic diseases in the United States that can affect you at any age.
The severity of the disease depends on the number of cysts that grow in the kidneys, their size, and how much the growth of cysts interferes with kidney function. If you are experiencing kidney problems and a parent has the disease or genetic testing shows positive results, Mount Sinai's team of experts can help you.
Our team of highly ranked nephrologists has the expertise to diagnose, manage, and treat all forms of PKD. In addition, we have one of the leading research labs in the nation dedicated to studying people with PKD.
The severity of the disease depends on the number of cysts that grow in the kidneys, their size, and how much the growth of cysts interferes with kidney function. If you are experiencing kidney problems and a parent has the disease or genetic testing shows positive results, Mount Sinai's team of experts can help you.
Our team of highly ranked nephrologists has the expertise to diagnose, manage, and treat all forms of PKD. In addition, we have one of the leading research labs in the nation dedicated to studying people with PKD.
What Is PKD?
As its name indicates, polycystic kidney disease involves many cysts forming on and in the kidneys. Cysts are fluid-filled sacs that can grow larger and increase in number over time, causing the kidneys to become enlarged. The cysts are not cancerous, but as kidneys become enlarged and overrun with cysts, you may lose kidney function, leading eventually to kidney failure.
In some cases, cysts may also develop in the liver and other parts of the body, causing serious complications in the brain and heart. Another common complication of the disease is high blood pressure.
Types of PKD
The most common type, representing approximately 90 percent of all cases of inherited PKD, is autosomal dominant polycystic kidney disease (ADPKD). With ADPKD, if one parent has the disease, there is a 50 percent chance for each offspring to develop it, too. This form of the disease typically starts in adulthood, but can develop in childhood.
Another. much less common type of inherited PKD is autosomal recessive polycystic kidney disease (ARPKD). With ARPKD, two parents must carry the gene, and even then, there is only a 25 percent chance that each offspring will have it too. This form of the disease typically starts close to birth, but can occur later in childhood.
Symptoms
The following symptoms may indicate that you need to be examined and possibly diagnosed and treated for polycystic kidney disease:
Blood in the urine
Headaches
High blood pressure
Kidney stones and infections
Pain in the back or side
Swelling of the abdomen and feeling full
Urinary tract infections
Diagnosis
To diagnose cysts in the kidney, we may use various imaging tests: ultrasound, computed tomography scan, and magnetic resonance imaging. This also allows us to see how much of your healthy kidney remains functional.
Treatments We Offer
Once we have a clear picture of your kidneys, we can address your needs. We bring together specialists who tailor your care by using minimally invasive surgery to remove infected or bleeding cysts, medical genetics, urology, cardiology, pediatrics, and nutrition, all to help you. While there is no cure for PKD, we use highly advanced treatments and preventive therapies to relieve many of your symptoms.
Mount Sinai nephrologists and collaborating specialists employ a full range of medical and support services throughout the courses of your treatment. We pride ourselves in caring for our PKD patients with:
Continuity of care. At all ages and stages of the disease, our nephrologists work in close collaboration with pediatric nephrologists to transition your children with PKD from pediatric care to adult PKD care.
Genetic counseling. Our genetics department specializes in evaluating and counseling you and your family with a history of PKD.
Transplantation. We work closely with our transplant team and may suggest that you get an evaluation for a kidney transplant. Our Recanati/Miller Transplantation Institute surgeons and physicians, along with specialized nurses and social workers, coordinate care before and after kidney transplantation.
In addition to our Mount Sinai Health System community of clinicians and researchers, we consult with the New York chapter of the PKD Foundation. We attend annual PKD conferences and participate in the Walk for PKD.
From CMAJ Group, Canadian Medical Association Journal
Use of vitamin D drops leading to kidney failure in a 54-year-old man
KEY POINTS
Vitamin D toxicity is rare, but clinicians must be aware of the risks of vitamin D use to limit complications related to hypercalcemia.
Calcium levels may get worse before getting better in patients even after cessation of supplements, as vitamin D is fat soluble.
Observational data and expert opinion suggest that glucocorticoids, ketoconazole and hydroxychloroquine are reasonable options to treat hypercalcemia related to vitamin D toxicity by decreasing the “active” 1, 25 dihydroxyvitamin D3 levels.
A 54-year-old man was referred urgently to the nephrology clinic by his family physician for suspected acute kidney injury, with a creatinine level of 376 μmol/L. He had recently returned from a trip to Southeast Asia, where he had spent extensive periods sunbathing (6–8 h/d) for 2 weeks. His medical history included hypertension, dyslipidemia and gout, for which he was taking perindopril 8 mg daily, rosuvastatin 10 mg daily, amlodipine 10 mg daily, indapamide 2.5 mg daily and febuxostat 80 mg daily.
On his return to Canada, the patient’s creatinine level had initially increased from his baseline of 100 μmol/L to 132 μmol/L. His family physician instructed him to discontinue his antihypertensive and diuretic agents temporarily on the premise that he had possible extracellular fluid depletion from the heat exposure. Despite this measure, on repeat measurement 4 weeks later, the patient’s creatinine level had risen to 376 μmol/L. During this 4-week period, he had not used nonsteroidal antiinflammatory drugs or new medications, had not been exposed to intravenous contrast and had no acute illnesses. Given that his creatinine level continued to rise rapidly with no clear etiology, the patient was referred to nephrology.
The patient’s family history included autosomal dominant polycystic kidney disease, with 2 first-degree relatives requiring dialysis before age 60. However, he had undergone radiographic screening with abdominal ultrasonography, which was negative for polycystic kidneys.
At the nephrology clinic, the patient’s blood pressure was 149/98 mm Hg, with no urgent clinical indications for dialysis. Renal ultrasonography showed normal-sized kidneys with no hydronephrosis or echogenicity. An incidental 1.2 cm bladder mass was seen on ultrasonography and was later diagnosed as noninvasive urothelial carcinoma. (This was treated with local excision with interval surveillance, requiring no chemotherapy.)
Urine studies at the patient’s initial nephrology visit did not show leukocytes, erythrocytes or protein. There were no cellular casts or crystals seen on urine microscopy. Results of serum and urine protein electrophoresis studies were negative. Complete blood count was normal. However, the patient’s serum calcium and parathyroid hormone (PTH) levels showed a non-PTH-mediated hypercalcemia (Box 1). Testing of 25-hydroxyvitamin D3 and 1,25 dihydroxyvitamin D3 levels was ordered. Imaging studies of the chest and abdomen were unremarkable.
On more detailed questioning, the patient mentioned that he was seeing a naturopathic specialist who had prescribed high doses of vitamin D, advising him to take 8 drops of a specific brand daily. He did not have a history of a fragility fracture or documented vitamin D deficiency. The recommended brand contained 500 IU per drop. Unknowingly, the patient obtained another vitamin D preparation that contained 1000 IU per drop. The patient was not counselled about toxicity risks and, over a period of 2.5 years, he took 8–12 drops of vitamin D daily, for a total daily dose of 8000–12 000 IU.
At the nephrology clinic, the patient’s measured 1,25 dihydroxyvitamin D3 level was 274 pmol/L and his 25-hydroxyvitamin D3 level was 241 nmol/L (Box 1). He was instructed to stop taking all vitamin D supplements and calcium-rich foods. His diuretics remained on hold, but one of his antihypertensive agents (amlodipine) was resumed after his second clinic visit. His 1,25 dihydroxyvitamin D3 and ionized calcium levels continued to increase (Figure 2). His only new symptom related to hypercalcemia was pruritus.
Given his worsening hypercalcemia and increased active vitamin D levels, we counselled the patient about starting glucocorticoid therapy. He was reluctant to start glucocorticoids, given concerns about potential weight gain. As an alternative, we offered oral hydroxychloroquine at 400 mg daily and counselled the patient on adverse effects, including retinal toxicity.
The patient’s calcium and vitamin D levels decreased after initiation of hydroxychloroquine. Almost 1 year after diagnosis, his calcium and vitamin D levels have returned to normal, but he is left with stage 3B (estimated glomerular filtration rate 34 mL/min/1.73m3) chronic kidney disease.
Discussion
Historically, reports have outlined the benefits of vitamin D in relation to bone health.1 Other purported benefits of vitamin D included nonskeletal outcomes, such as cardiovascular benefit, fall prevention, and reduction of infections and malignancies.2 However, an umbrella review of systematic reviews and meta-analyses did not show that vitamin D reduces primary fracture risk or convincingly improves other nonskeletal health outcomes.3 Furthermore, a recent review for the US Preventive Services Task Force showed no benefit of vitamin D in preventing primary fracture in those without known deficiency, osteoporosis or prior fracture.4
In its 2010 guideline, Osteoporosis Canada recommended vitamin D supplementation of 10–25 μg (400–1000 IU) for most low-risk adults under the age of 50 years to achieve serum levels of 25-hydroxyvitamin D3 greater than 75 nmol/L (level 3 evidence),1 arguing that the potential benefits outweigh risks. A daily vitamin D intake of 20–50 μg (800–2000 IU) is recommended for high-risk and older adults (level 2 evidence).1
Mechanism of vitamin D toxicity
Although vitamin D toxicity is rare owing to a large therapeutic range, its widespread availability in various over-the-counter formulations may pose a substantial risk to uninformed patients. After consumption, vitamin D is carried to the liver where it undergoes hydroxylation and is activated by either microsomal CYP2R1 or mitochondrial CYP27A1 to 25-hydroxyvitamin D3.5,6 The resulting 25-hydroxyvitamin D3 binds to the vitamin D binding protein and is carried to the kidneys for further 1α-hydroxylation by CYP27B1 to produce 1,25 dihydroxyvitamin D3.6 This 1,25 dihydroxyvitamin D3 is transported to target cells and enters the nucleus of the vitamin D receptor, leading to an upregulation in gene expression. Although it is transported by vitamin D binding protein, 1,25 dihydroxyvitamin D3 has a lower affinity to binding relative to 25-hydroxyvitamin D3 and its metabolites.5 A leading hypothesis suggests that an oversaturation of the vitamin D binding protein causes an increase in free active Vitamin D (1,25 dihydroxyvitamin D3), resulting in hypercalcemia.
CYP24A1 plays an important role in the deactivation of 1,25 dihydroxyvitamin D3 to calcitroic acid.6CYP24A1 also breaks down precursor 25-hydroxyvitamin D3 to 24,25-dihydroxyvitamin D3. Loss-of-function mutations in CYP24A1 have been associated with hypercalcemia because of increased vitamin D sensitivity.6 Although we did not perform genetic testing on our patient, it is plausible that he had a CYP24A1 mutation, increasing his susceptibility to vitamin D toxicity.
Manifestations of toxicity
Toxicity can occur over a short period in patients ingesting large doses of vitamin D, either intentionally or inadvertently.7 The literature supports that doses greater than 10 000 IU per day for several months may lead to toxicity (> 200 nmol/L of 25-hydroxyvitamin D3).8 However, differences in patient characteristics such as malabsorption and mutations in CYP24A1 can lead to substantial variation in doses required for toxicity to occur.
Patients may present with symptoms involving the central nervous system and gastrointestinal, genitourinary and cardiovascular systems. Central nervous system manifestations include lethargy, hypotonia, hyporeflexia, confusion and coma. Gastrointestinal symptoms include nausea, vomiting, pancreatitis and constipation; cardiovascular symptoms of toxicity, such as hypertension, arrhythmias and QT segment shortening, may also occur.9 Genitourinary symptoms include polyuria, nephrocalcinosis and renal failure. The symptomatology associated with vitamin D toxicity underscores the suggestion that hypercalcemia may be responsible for most symptoms seen.7Sustained hypercalcemia can also lead to dysregulation of calcium–phosphate homeostasis leading to PTH suppression and impaired bone turnover.3,5
Importantly, patients may be asymptomatic, delaying diagnosis, and abnormalities related to vitamin D toxicity may be detected only incidentally.
In terms of renal involvement, hypercalcemia can cause kidney injury both acutely and chronically. Hypercalcemia can cause acute kidney injury primarily by 2 mechanisms: afferent arteriolar constriction and intravascular volume depletion from a diuretic effect through activation of calcium-sensing receptor at the sodium–chloride cotransporter in the loop of Henle.10 Our patient’s acute kidney injury was likely worsened by volume depletion from diuretic use, prolonged heat exposure and preexisting hypercalcemia from vitamin D toxicity. We saw some improvement in his renal function as his calcium levels decreased, by discontinuing his vitamin D supplements and temporarily holding his diuretics and antihypertensive agents. However, we believe that our patient developed chronic disease as shown by nephrosclerosis on renal biopsy (Figure 1).
Management of toxicity
In cases where vitamin D toxicity is suspected, patients should have their medications — prescribed and over-the-counter — carefully reviewed. Initial strategies to reduce vitamin D levels should focus on reducing dietary or supplementary sources. Although there is currently no evidence from large trials, observational data suggest that clinicians may consider strategies to reduce active vitamin D levels if hypercalcemia persists, through inhibition of 1α-hydroxylase activity (Figure 3).11,12 If patients are asymptomatic, clinicians may choose to monitor levels expectantly, as vitamin D is very fat soluble and levels may take some time to return to normal.
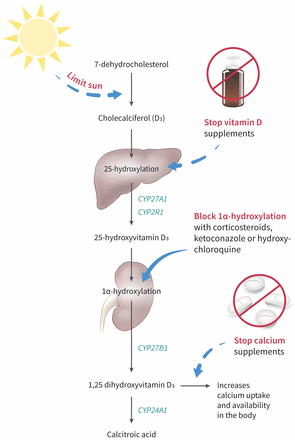
Several medications have been used successfully to treat hypercalcemia by reducing the active form of vitamin D. Glucocorticoids, ketoconazole and hydroxychloroquine have all been used in cases of hypercalcemia related to sarcoidosis.1,9 Expert opinion suggests that these medications reduce 1α-hydroxylase activity and may be used to manage hypercalcemia by reducing 1,25 dihydroxyvitamin D3 levels.11,12 Given that our patient was reluctant to use glucocorticoid treatment, we used hydroxychloroquine as an alternative to decrease his 1,25 dihydroxyvitamin D3 levels and, in turn, his calcium levels.
Our experience informs us that patients and clinicians should be better informed about the risks regarding the unfettered use of vitamin D. Given new findings from the US Preventive Services Task Force,4 current Canadian guidelines regarding its use in low-risk individuals should be revisited.
Patients with CYP24A1 mutations may be at an increased risk of vitamin D toxicity, and clinicians can consider genetic testing if vitamin D toxicity develops with doses less than 10 000 IU per day. Although vitamin D toxicity is rare, early recognition may prevent chronic complications related to hypercalcemia. In patients who are symptomatic, cessation of supplements along with treatment with glucocorticoids is suggested. In cases where glucocorticoid therapy is not preferred or is contraindicated, ketoconazole or hydroxychloroquine are reasonable alternatives.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Vitamin D toxicity is rare, but clinicians must be aware of the risks of vitamin D use to limit complications related to hypercalcemia.
Calcium levels may get worse before getting better in patients even after cessation of supplements, as vitamin D is fat soluble.
Observational data and expert opinion suggest that glucocorticoids, ketoconazole and hydroxychloroquine are reasonable options to treat hypercalcemia related to vitamin D toxicity by decreasing the “active” 1, 25 dihydroxyvitamin D3 levels.
A 54-year-old man was referred urgently to the nephrology clinic by his family physician for suspected acute kidney injury, with a creatinine level of 376 μmol/L. He had recently returned from a trip to Southeast Asia, where he had spent extensive periods sunbathing (6–8 h/d) for 2 weeks. His medical history included hypertension, dyslipidemia and gout, for which he was taking perindopril 8 mg daily, rosuvastatin 10 mg daily, amlodipine 10 mg daily, indapamide 2.5 mg daily and febuxostat 80 mg daily.
On his return to Canada, the patient’s creatinine level had initially increased from his baseline of 100 μmol/L to 132 μmol/L. His family physician instructed him to discontinue his antihypertensive and diuretic agents temporarily on the premise that he had possible extracellular fluid depletion from the heat exposure. Despite this measure, on repeat measurement 4 weeks later, the patient’s creatinine level had risen to 376 μmol/L. During this 4-week period, he had not used nonsteroidal antiinflammatory drugs or new medications, had not been exposed to intravenous contrast and had no acute illnesses. Given that his creatinine level continued to rise rapidly with no clear etiology, the patient was referred to nephrology.
The patient’s family history included autosomal dominant polycystic kidney disease, with 2 first-degree relatives requiring dialysis before age 60. However, he had undergone radiographic screening with abdominal ultrasonography, which was negative for polycystic kidneys.
At the nephrology clinic, the patient’s blood pressure was 149/98 mm Hg, with no urgent clinical indications for dialysis. Renal ultrasonography showed normal-sized kidneys with no hydronephrosis or echogenicity. An incidental 1.2 cm bladder mass was seen on ultrasonography and was later diagnosed as noninvasive urothelial carcinoma. (This was treated with local excision with interval surveillance, requiring no chemotherapy.)
Urine studies at the patient’s initial nephrology visit did not show leukocytes, erythrocytes or protein. There were no cellular casts or crystals seen on urine microscopy. Results of serum and urine protein electrophoresis studies were negative. Complete blood count was normal. However, the patient’s serum calcium and parathyroid hormone (PTH) levels showed a non-PTH-mediated hypercalcemia (Box 1). Testing of 25-hydroxyvitamin D3 and 1,25 dihydroxyvitamin D3 levels was ordered. Imaging studies of the chest and abdomen were unremarkable.
On more detailed questioning, the patient mentioned that he was seeing a naturopathic specialist who had prescribed high doses of vitamin D, advising him to take 8 drops of a specific brand daily. He did not have a history of a fragility fracture or documented vitamin D deficiency. The recommended brand contained 500 IU per drop. Unknowingly, the patient obtained another vitamin D preparation that contained 1000 IU per drop. The patient was not counselled about toxicity risks and, over a period of 2.5 years, he took 8–12 drops of vitamin D daily, for a total daily dose of 8000–12 000 IU.
At the nephrology clinic, the patient’s measured 1,25 dihydroxyvitamin D3 level was 274 pmol/L and his 25-hydroxyvitamin D3 level was 241 nmol/L (Box 1). He was instructed to stop taking all vitamin D supplements and calcium-rich foods. His diuretics remained on hold, but one of his antihypertensive agents (amlodipine) was resumed after his second clinic visit. His 1,25 dihydroxyvitamin D3 and ionized calcium levels continued to increase (Figure 2). His only new symptom related to hypercalcemia was pruritus.
Given his worsening hypercalcemia and increased active vitamin D levels, we counselled the patient about starting glucocorticoid therapy. He was reluctant to start glucocorticoids, given concerns about potential weight gain. As an alternative, we offered oral hydroxychloroquine at 400 mg daily and counselled the patient on adverse effects, including retinal toxicity.
The patient’s calcium and vitamin D levels decreased after initiation of hydroxychloroquine. Almost 1 year after diagnosis, his calcium and vitamin D levels have returned to normal, but he is left with stage 3B (estimated glomerular filtration rate 34 mL/min/1.73m3) chronic kidney disease.
Discussion
Historically, reports have outlined the benefits of vitamin D in relation to bone health.1 Other purported benefits of vitamin D included nonskeletal outcomes, such as cardiovascular benefit, fall prevention, and reduction of infections and malignancies.2 However, an umbrella review of systematic reviews and meta-analyses did not show that vitamin D reduces primary fracture risk or convincingly improves other nonskeletal health outcomes.3 Furthermore, a recent review for the US Preventive Services Task Force showed no benefit of vitamin D in preventing primary fracture in those without known deficiency, osteoporosis or prior fracture.4
In its 2010 guideline, Osteoporosis Canada recommended vitamin D supplementation of 10–25 μg (400–1000 IU) for most low-risk adults under the age of 50 years to achieve serum levels of 25-hydroxyvitamin D3 greater than 75 nmol/L (level 3 evidence),1 arguing that the potential benefits outweigh risks. A daily vitamin D intake of 20–50 μg (800–2000 IU) is recommended for high-risk and older adults (level 2 evidence).1
Mechanism of vitamin D toxicity
Although vitamin D toxicity is rare owing to a large therapeutic range, its widespread availability in various over-the-counter formulations may pose a substantial risk to uninformed patients. After consumption, vitamin D is carried to the liver where it undergoes hydroxylation and is activated by either microsomal CYP2R1 or mitochondrial CYP27A1 to 25-hydroxyvitamin D3.5,6 The resulting 25-hydroxyvitamin D3 binds to the vitamin D binding protein and is carried to the kidneys for further 1α-hydroxylation by CYP27B1 to produce 1,25 dihydroxyvitamin D3.6 This 1,25 dihydroxyvitamin D3 is transported to target cells and enters the nucleus of the vitamin D receptor, leading to an upregulation in gene expression. Although it is transported by vitamin D binding protein, 1,25 dihydroxyvitamin D3 has a lower affinity to binding relative to 25-hydroxyvitamin D3 and its metabolites.5 A leading hypothesis suggests that an oversaturation of the vitamin D binding protein causes an increase in free active Vitamin D (1,25 dihydroxyvitamin D3), resulting in hypercalcemia.
CYP24A1 plays an important role in the deactivation of 1,25 dihydroxyvitamin D3 to calcitroic acid.6CYP24A1 also breaks down precursor 25-hydroxyvitamin D3 to 24,25-dihydroxyvitamin D3. Loss-of-function mutations in CYP24A1 have been associated with hypercalcemia because of increased vitamin D sensitivity.6 Although we did not perform genetic testing on our patient, it is plausible that he had a CYP24A1 mutation, increasing his susceptibility to vitamin D toxicity.
Manifestations of toxicity
Toxicity can occur over a short period in patients ingesting large doses of vitamin D, either intentionally or inadvertently.7 The literature supports that doses greater than 10 000 IU per day for several months may lead to toxicity (> 200 nmol/L of 25-hydroxyvitamin D3).8 However, differences in patient characteristics such as malabsorption and mutations in CYP24A1 can lead to substantial variation in doses required for toxicity to occur.
Patients may present with symptoms involving the central nervous system and gastrointestinal, genitourinary and cardiovascular systems. Central nervous system manifestations include lethargy, hypotonia, hyporeflexia, confusion and coma. Gastrointestinal symptoms include nausea, vomiting, pancreatitis and constipation; cardiovascular symptoms of toxicity, such as hypertension, arrhythmias and QT segment shortening, may also occur.9 Genitourinary symptoms include polyuria, nephrocalcinosis and renal failure. The symptomatology associated with vitamin D toxicity underscores the suggestion that hypercalcemia may be responsible for most symptoms seen.7Sustained hypercalcemia can also lead to dysregulation of calcium–phosphate homeostasis leading to PTH suppression and impaired bone turnover.3,5
Importantly, patients may be asymptomatic, delaying diagnosis, and abnormalities related to vitamin D toxicity may be detected only incidentally.
In terms of renal involvement, hypercalcemia can cause kidney injury both acutely and chronically. Hypercalcemia can cause acute kidney injury primarily by 2 mechanisms: afferent arteriolar constriction and intravascular volume depletion from a diuretic effect through activation of calcium-sensing receptor at the sodium–chloride cotransporter in the loop of Henle.10 Our patient’s acute kidney injury was likely worsened by volume depletion from diuretic use, prolonged heat exposure and preexisting hypercalcemia from vitamin D toxicity. We saw some improvement in his renal function as his calcium levels decreased, by discontinuing his vitamin D supplements and temporarily holding his diuretics and antihypertensive agents. However, we believe that our patient developed chronic disease as shown by nephrosclerosis on renal biopsy (Figure 1).
Management of toxicity
In cases where vitamin D toxicity is suspected, patients should have their medications — prescribed and over-the-counter — carefully reviewed. Initial strategies to reduce vitamin D levels should focus on reducing dietary or supplementary sources. Although there is currently no evidence from large trials, observational data suggest that clinicians may consider strategies to reduce active vitamin D levels if hypercalcemia persists, through inhibition of 1α-hydroxylase activity (Figure 3).11,12 If patients are asymptomatic, clinicians may choose to monitor levels expectantly, as vitamin D is very fat soluble and levels may take some time to return to normal.

Several medications have been used successfully to treat hypercalcemia by reducing the active form of vitamin D. Glucocorticoids, ketoconazole and hydroxychloroquine have all been used in cases of hypercalcemia related to sarcoidosis.1,9 Expert opinion suggests that these medications reduce 1α-hydroxylase activity and may be used to manage hypercalcemia by reducing 1,25 dihydroxyvitamin D3 levels.11,12 Given that our patient was reluctant to use glucocorticoid treatment, we used hydroxychloroquine as an alternative to decrease his 1,25 dihydroxyvitamin D3 levels and, in turn, his calcium levels.
Our experience informs us that patients and clinicians should be better informed about the risks regarding the unfettered use of vitamin D. Given new findings from the US Preventive Services Task Force,4 current Canadian guidelines regarding its use in low-risk individuals should be revisited.
Patients with CYP24A1 mutations may be at an increased risk of vitamin D toxicity, and clinicians can consider genetic testing if vitamin D toxicity develops with doses less than 10 000 IU per day. Although vitamin D toxicity is rare, early recognition may prevent chronic complications related to hypercalcemia. In patients who are symptomatic, cessation of supplements along with treatment with glucocorticoids is suggested. In cases where glucocorticoid therapy is not preferred or is contraindicated, ketoconazole or hydroxychloroquine are reasonable alternatives.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Dr Itua cure my HIV, I have been a ARV Consumption for 10 years. i have been in pains until i came across Dr Itua on blogs site.I emailed him about my details of my HIV and my location i explained every thing to him and he told me that there is nothing to be scared of that he will cured me, he gave me guarantee,He ask me to pay for items fees so when i'm cured I will show gratitude I did and giving testimony of his healing herbs is what I'm going to do for the rest of you out there having HIV and other disease can see the good work of Dr Itua.I received his herbal medicine through EMS Courier service who delivered to my post office within 5 working days.Dr Itua is an honest man and I appreciate him for his good work.My GrandMa called him to appreciate him and rest of my friends did too,Is a joy to me that I'm free of taking Pills and having that fat belle is a nightmare.you will understand what i'm talking about if you have same problem I was having then not now though.I'm free and healthy Big Thanks To Dr Itua Herbal Center.I have his calendar too that he recently sent me,He Cure all kind disease Like,Cancer,Weak Erection,Wart Remover,Hpv,Herpes,Alzheimer’s disease,Bechet’s disease,Crohn’s disease
ReplyDelete,Cushing’s disease,Heart failure,Multiple Sclerosis,Hypertension,Fibromyalgia,Hiv,Hepatitis B,Liver/Kidney Inflamatory,Epilepsy,Blood Cancer,Prostate Cancer,Colo-Rectal Cancer,Brain Cancer,Lung Cancer,Infertility,Parkinson's disease,Schizophrenia,Lung Cancer,Breast Cancer,Colo-Rectal Cancer,Blood Cancer,Prostate Cancer,siva.Fatal Familial Insomnia Factor V Leiden Mutation ,Epilepsy Dupuytren's disease,Desmoplastic small-round-cell tumor Diabetes ,Coeliac disease,Creutzfeldt–Jakob disease,Cerebral Amyloid Angiopathy, Ataxia,Arthritis,measles, tetanus, whooping cough, tuberculosis, polio and diphtheriaAmyotrophic Lateral Scoliosis,Fibromyalgia,Fluoroquinolone Toxicity
Syndrome Fibrodysplasia Ossificans ProgresSclerosis,Seizures,Alzheimer's disease,Adrenocortical carcinoma.Asthma,Allergic diseases.Hiv_ Aids,Herpe ,Copd,Glaucoma., Cataracts,Macular degeneration,Cardiovascular disease,Lung disease.Enlarged prostate,Osteoporosis.Alzheimer's disease,
Dementia.Fibroid,Diabetes,Dercum,Copd ,and also Bring back Ex Lover Back..Here his Contact .drituaherbalcenter@gmail.com Or Whats_app Number +2348149277967