From Medical Daily, By Leian Naduma
Renal or kidney pain is associated with several malfunctions of the organs or recurring infection. Sometimes, it is a mere urinary tract infection that can be remedied by lifestyle changes or prescription medication. Other times, it is a symptom of a serious mental condition. Here are the most common signs, symptoms and causes to help you identify if what you are going through is a mild or serious case of kidney pain.
Your kidneys are bean-shaped organs located on either side of your spine. They filter the blood and balance the number of fluids and electrolytes in your body. The level of pain you experience also depends on whether the cause is prerenal or related to another organ located near it, intrinsic or caused by the kidneys themselves or postrenal, which is typically due to an obstruction below the organs. Thus, the common triggers of kidney pain are an infection, obstruction, growth or trauma, as per VeryWell Health.
Trauma
Kidneys are placed at a vulnerable position in the abdomen which may easily be affected by a blunt force impact or a penetrating wound. If you have abdominal injuries, there is a 10 percent chance that you will also sustain damage to your kidneys. Events such as physical assaults and vehicular accidents result in renal trauma. You can distinguish this cause from others by pressing the kidney area and note a painful sensation. It may also be associated with fever, hematuria, urinary retention, rapid heart rate, decreased alertness and abdominal swelling. These symptoms require emergency treatment.
Renal Obstruction
This is a result of urinary blockage and it is an intrinsic type of kidney pain. The renal obstruction causes unilateral or bilateral pain due to affected ureters. This type of pain is also called obstructive uropathy which is caused by kidney stones, bladder stones, urinary tract infection, an enlarged prostate, pregnancy, long-term catheterization, a blood clot in the kidney, nerve-related bladder weakness, cancer, or vesicoureteral reflux. These cause your kidneys to swell and is referred to as hydronephrosis. You are most likely to experience pain in the groin, flak or abdominal area and may be associated with urinary urgency, fever, nausea and dysuria. Kidney stones cause higher pain levels while the others are gradual when left untreated.
Growth or Cysts
Renal tumors also cause pain and its intensity depends on the growth. The three most common growth abnormalities are renal adenoma, renal cell carcinoma and polycystic kidney disease (PKD). Larger ones do not cause pain until they disturb the architecture of the kidney. When this happens, the pain is persistent and worsens over time. If the tumor is cancerous, you may also experience unexplained weight loss, which suggests an advanced malignancy. PKD is also associated with headaches, high blood pressure, abdominal pain and swelling, hematuria and renal failure.
Infection
Kidney infection is treated with prescription antibiotics. This is caused by viral or fungal exposure that adversely affects the body’s immune system. Patients who have advanced HIV or underwent organ transplants are more vulnerable to this condition.
Prevention And Cure
As per Medicine.Net, most kidney pains can be relieved by drugs like ibuprofen, ketorolac and acetaminophen, which are common medications for pain. Antibiotics are recommended by doctors in infections while those who have kidney stones may need surgery.
Kidney pains caused by infections can be prevented by drinking sufficient amounts of water per day, wearing loose pants, following proper hygiene and refraining from holding your urine for long periods. Kidney stones and abdominal cancers, on the other hand, may be prevented by eating a healthy diet and avoiding salty and fatty foods.
From Health Care Finance, by Jeff Lagasse, Associate Editor
Modifications to Medicare rules could support care innovation for dialysis
Your kidneys are bean-shaped organs located on either side of your spine. They filter the blood and balance the number of fluids and electrolytes in your body. The level of pain you experience also depends on whether the cause is prerenal or related to another organ located near it, intrinsic or caused by the kidneys themselves or postrenal, which is typically due to an obstruction below the organs. Thus, the common triggers of kidney pain are an infection, obstruction, growth or trauma, as per VeryWell Health.
Trauma
Kidneys are placed at a vulnerable position in the abdomen which may easily be affected by a blunt force impact or a penetrating wound. If you have abdominal injuries, there is a 10 percent chance that you will also sustain damage to your kidneys. Events such as physical assaults and vehicular accidents result in renal trauma. You can distinguish this cause from others by pressing the kidney area and note a painful sensation. It may also be associated with fever, hematuria, urinary retention, rapid heart rate, decreased alertness and abdominal swelling. These symptoms require emergency treatment.
Renal Obstruction
This is a result of urinary blockage and it is an intrinsic type of kidney pain. The renal obstruction causes unilateral or bilateral pain due to affected ureters. This type of pain is also called obstructive uropathy which is caused by kidney stones, bladder stones, urinary tract infection, an enlarged prostate, pregnancy, long-term catheterization, a blood clot in the kidney, nerve-related bladder weakness, cancer, or vesicoureteral reflux. These cause your kidneys to swell and is referred to as hydronephrosis. You are most likely to experience pain in the groin, flak or abdominal area and may be associated with urinary urgency, fever, nausea and dysuria. Kidney stones cause higher pain levels while the others are gradual when left untreated.
Growth or Cysts
Renal tumors also cause pain and its intensity depends on the growth. The three most common growth abnormalities are renal adenoma, renal cell carcinoma and polycystic kidney disease (PKD). Larger ones do not cause pain until they disturb the architecture of the kidney. When this happens, the pain is persistent and worsens over time. If the tumor is cancerous, you may also experience unexplained weight loss, which suggests an advanced malignancy. PKD is also associated with headaches, high blood pressure, abdominal pain and swelling, hematuria and renal failure.
Infection
Kidney infection is treated with prescription antibiotics. This is caused by viral or fungal exposure that adversely affects the body’s immune system. Patients who have advanced HIV or underwent organ transplants are more vulnerable to this condition.
Prevention And Cure
As per Medicine.Net, most kidney pains can be relieved by drugs like ibuprofen, ketorolac and acetaminophen, which are common medications for pain. Antibiotics are recommended by doctors in infections while those who have kidney stones may need surgery.
Kidney pains caused by infections can be prevented by drinking sufficient amounts of water per day, wearing loose pants, following proper hygiene and refraining from holding your urine for long periods. Kidney stones and abdominal cancers, on the other hand, may be prevented by eating a healthy diet and avoiding salty and fatty foods.
Medicare spends approximately $35 billion annually on care for beneficiaries with end-stage renal disease.
In a commentary published in the American Journal of Kidney Diseases, public health researchers suggest adjustments to recently proposed rule changes on how Medicare pays for dialysis services.
Medicare spends approximately $35 billion annually on care for beneficiaries with end-stage renal disease, or kidney failure. That's more than 7 percent of Medicare's total paid claims. Over half a million people receive regular dialysis treatments to manage this condition, with treatment costs averaging about $85,000 a year, according to the study.
IMPACT
Rule changes were proposed about a year ago that would limit the number of dialysis treatments per week that would be paid for by Medicare. Interest groups including nephrologists and patients themselves were concerned that this would limit patient access to innovative treatment options, such as frequent hemodialysis.
Under the current system, Medicare covers three hemodialysis treatments weekly per patient, but it will often pay for additional treatments when the treating nephrologist provides sufficient medical justification.
The recently proposed rule changes would limit such additional payments to exceptional circumstances -- for example, patients with temporary, acute kidney treatment needs. Although nephrologists would not be prevented from providing any "extra" treatments they believe are needed, they would typically bear the costs of doing so.
The researchers discussed the limitations of the current evidence on frequent dialysis treatment, which to date has yielded mixed conclusions. Their suggested changes to Medicare's dialysis payment system were designed to account for these limitations and give Medicare the flexibility to further modify the system in the future as new evidence comes to light.
Under their main proposal, Medicare would establish a new, separate prospective payment system for frequent hemodialysis treatment.
In that way, they said, nephrologists would have greater clarity about how their dialysis care would be paid for, and free them up to pinpoint better ways to treat their patients.
THE TREND
In the first three years of Medicaid expansion due to the Affordable Care Act, the number of patients with end-stage kidney disease who died within a year of starting dialysis decreased in states that expanded Medicaid, compared to non-expansion states, found research published in October.
The adjusted absolute reduction in mortality in expansion states versus non-expansion states was 0.6 percentage points. Since end-stage renal disease affects more than 100,000 Americans each year, 0.6 percentage points equals hundreds of deaths annually.
PKD Research
Select Science
Modeling Kidney Disease with Bioengineered Kidney Organoids

Kidney organoid pioneer, Dr. Benjamin Freedman, explains the bioengineering, generation and application of 3D cell culture in studying kidney disease.
Kidney disease, prevalent in about 14% of the American population, lacks much-needed early interventions to prevent disease progression. Current methods that address kidney disease in a more chronic stage endure additional complexities: for example, artificial kidney devices don’t function as well as a human kidney, and renal transplant procedures need the support of anti-rejection medication.
In this article, we interview Dr. Benjamin Freedman, Assistant Professor at the University of Washington, whose goal is to understand kidney disease in its early stages. “In the medical practice, there's a lot of focus on the end stages of kidney disease and on managing that chronic disease. We are more interested in the early stages and all the different things that can go wrong early on,” says Freedman.
A pioneer in the field of kidney organoids, Freedman chose the 3D cell culture system to study kidney disease. “Organoids are great for this because they can show signs of disease,” Freedman explains. “We can then intervene by treating them with any compound that we're interested in.” A robust model for mimicking disease in vitro, kidney organoids didn’t even exist until a few years ago.
Freedman was the first scientist in the western hemisphere to generate a kidney organoid from pluripotent stem cells. “That was a very exciting moment,” Freedman recalls. “As soon as I saw these structures, I knew that there was something interesting about them. I had all the tools at my disposal to test whether they were indeed kidney.”
And they were.
Bioengineering kidney organoids
Developing and maintaining kidney organoids requires bioengineering capabilities. While the organoids grow and self-assemble from stem cells, their in vitro development deprives them of the vascular perfusion otherwise available in vivo. Tiny microfluidic tubules pass through the kidneys in our bodies, enabling the inlet and outlet of fluid, a resource that kidney organoids grown on a dish don’t receive.
“The kidneys in the body get about 20–25% of the cardiac output at any time point,” explains Freedman. “One thing we're missing in an organoid structure is the ability to perfuse the organoids and the tiny tubules.”
Freedman’s lab has a solution to this problem. “We're trying to make the kidney organoid 2.0, which will incorporate not just the stem cells and their natural ability to form the structures but will also impose a bioengineering design on top of those structures to enable them to really form the very complex types of functional tubules that are found in the body.”
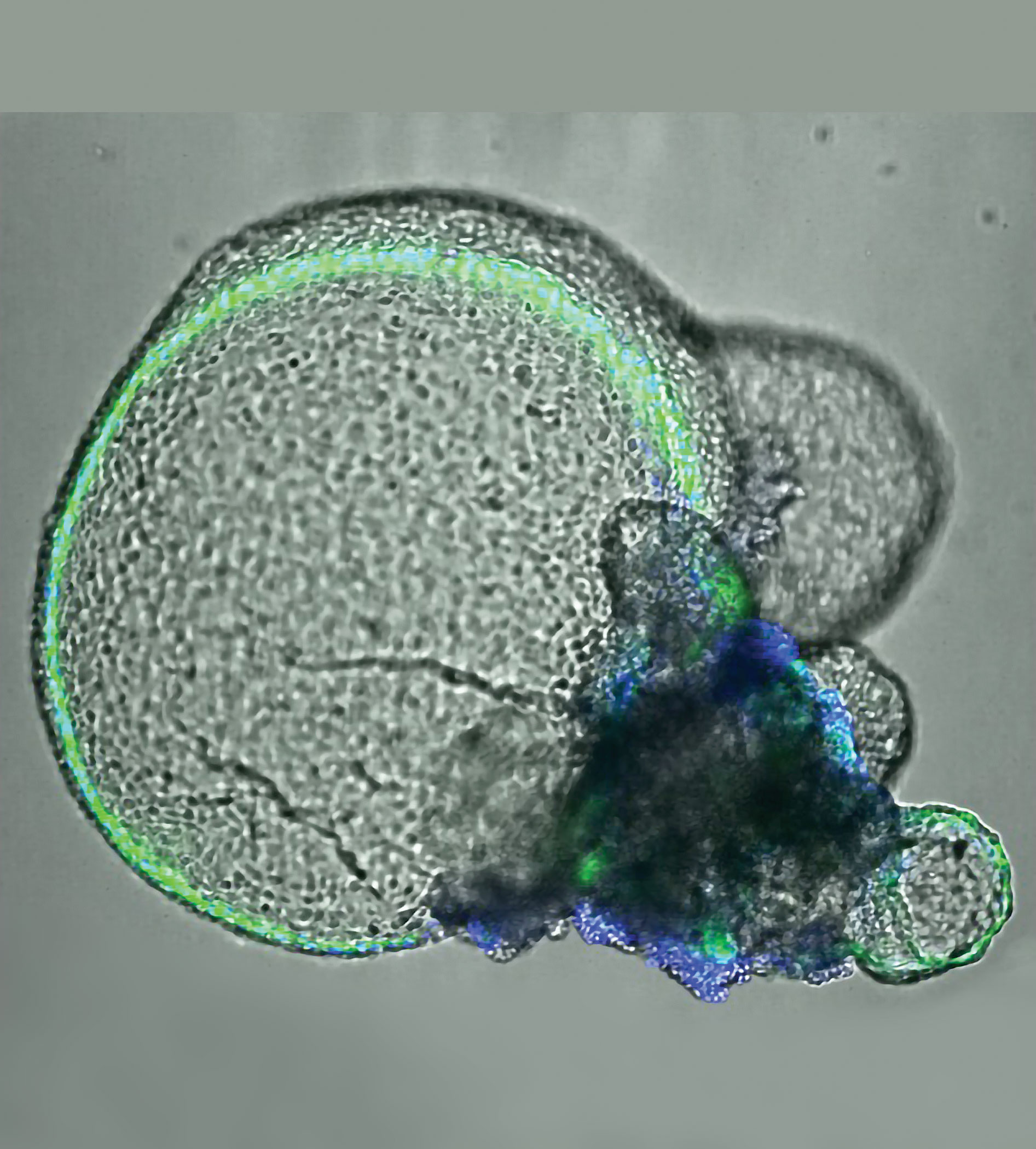
 Kidney organoids cultured in the Freedman lab. Images from left to right; blood vessels joining kidney organoids; grown from reprogrammed skin cells; showing cyst growth from tubules; kidney podocytes. Image courtesy of the Freedman lab.
Kidney organoids cultured in the Freedman lab. Images from left to right; blood vessels joining kidney organoids; grown from reprogrammed skin cells; showing cyst growth from tubules; kidney podocytes. Image courtesy of the Freedman lab.
Mimicking in vivo environments
The kidney organoid 2.0 grows on a kidney-on-a-chip device incorporated with extracellular matrix in scaffolded arrangements. By seeding the stem cell-derived kidney cells on this matrix-coated chip, the cells can be grown into controlled shapes and arrangements. “It's a fusion between stem cell and bioengineering fields,” explains Freedman. “The chip, about the size of a credit card, has a tiny tubule structure through which we can perfuse liquid. We can then observe the absorption of solutes such as glucose and ions and see how they're transported from one side of the tubule to the other side.”
The procedure to generate organoids uses a thin layer of Corning® Matrigel® matrix at the bottom of the tissue culture dish. The cells are plated on top of this layer, followed by a second thin layer of Matrigel. “This enables the cells to fold up and create more three-dimensional types of structures which are about 200 microns in diameter,” says Freedman. “These contain all the key lineages of the kidney tissue that we're interested in.”
The Matrigel matrix forms a crucial component of the kidney organoid generation process from pluripotent stem cells. “Matrigel matrix helps the cells to survive. They need a malleable support that they can grow into and form more three-dimensional structures in; something that they can actually change and remodel as they're growing,” Freedman explains.
For the kidney-on-a-chip protocol, the Freedman lab uses a relatively strong and stiff extracellular matrix. “We use Corning Collagen I, a highly concentrated form of collagen because these gels need to be stiff for the cells to take on the right shape that we’re interested in,” says Freedman. “If it’s too soft, the whole thing just falls apart, especially because we have fluid running through it.”
Alternatively, after the organoids have formed, they are placed inside larger Corning Collagen I droplets to stimulate the cells to grow out and populate. “This way, we study the ability of these cells to migrate out of the organoid, which has ramifications for certain disease processes,” says Freedman. “The tubular cells, which are normally sedentary, can actually migrate out when you give them an environment like that.”
Better detached than attached
One of the projects in the Freedman lab involves studying polycystic kidney disease using the kidney organoid model. “In polycystic kidney disease, the tubules, which are normally very narrow, swell up to form large balloon-life structures. They eventually crowd out and destroy all the healthy kidney tissue,” explains Freedman. “We’ve been able to get this to actually happen in our kidney organoids by mutating a gene in the stem cells and derive organoids that are mutants. The organoids swell just like the kidney tubules in the disease.”
This swelling process, however, depends on the microenvironment the organoid finds itself in. Freedman adds: “If you grow the organoids in a condition where they’re essentially floating in media, then it accelerates and exacerbates the disease process.” The lab uses Corning ultra-low attachment plates to grow the organoids detached. “They make these big balloon-like cyst structures very well in the ultra-low attachment plates,” notes Freedman. “They don’t do as well when grown in normal tissue culture conditions.”
The next big thing for 3D cell culture
High-throughput chemical and genetic screening are soon making their way into 3D cell culture biology. “We’re going to move from a phase where we study one drug and its effects at a time to a stage where we’re interrogating the organoids with thousands of compounds,” says Freedman.
“This is something you can’t do in the mammalian animal model, so it’s an exciting time for unbiased discovery using organoids. The big data phase is upon us.”
Pioneering Breakthrough: Unmanned Aircraft Delivers Organ for Successful Kidney Transplant in Maryland
COLLEGE PARK and BALTIMORE, Md., April 26, 2019 /PRNewswire/ -- In a first-ever advancement in human medicine and aviation technology, a University of Maryland (UMD) unmanned aircraft has delivered a donor kidney to surgeons at the University of Maryland Medical Center (UMMC) in Baltimore for successful transplantation into a patient with kidney failure. This successful demonstration illustrates the potential of unmanned aircraft systems (UAS) for providing organ deliveries that, in many cases, could be faster, safer, and more widely available than traditional transport methods.
The momentous flight was a collaboration between aviation and engineering experts at the University of Maryland; transplant physicians and researchers at the University of Maryland School of Medicine (UMSOM) in Baltimore; and collaborators at the Living Legacy Foundation of Maryland.
"This whole thing is amazing. Years ago, this was not something that you would think about," said the kidney recipient, a 44-year-old Baltimore resident who spent eight years on dialysis before undergoing the transplant procedure.The patient was discharged from UMMC on Tuesday.
Maryland faculty and researchers believe this prototype organ transport blazes a trail for the use of UAS to expand access to donated organs, improving outcomes for more people in need of organ transplants.
"This history-making flight not only represents a breakthrough from a technological point of view, but provides an exemplary demonstration of how engineering expertise and ingenuity ultimately serve human needs—in this case, the need to improve the reliability and efficiency of organ delivery to hospitals conducting transplant surgery," said Darryll J. Pines, Ph.D., UMD, dean of the A. James Clark School of Engineering and Nariman Farvardin Professor of Aerospace Engineering. "As astonishing as this breakthrough is from a purely engineering point of view, there's a larger purpose at stake. It's ultimately not about the technology; it's about enhancing human life."
Added Joseph Scalea, MD, assistant professor of surgery at UMSOM, project lead, and one of the surgeons who performed the transplant at UMMC, "As a result of the outstanding collaboration among surgeons, engineers, the Federal Aviation Administration (FAA), organ procurement specialists, pilots, nurses, and, ultimately, the patient, we were able to make a pioneering breakthrough in transplantation."
The many technological firsts of this effort include: a specially designed, high-tech apparatus for maintaining and monitoring a viable human organ; a custom-built UAS with eight rotors and multiple powertrains to ensure consistently reliable performance, even in the case of a possible component failure; the use of a wireless "mesh" network to control the UAS, monitor aircraft status, and provide communications for the ground crew at multiple locations; and aircraft operating systems that combined best practices from both UAS and organ transport standards.
"We had to create a new system that was still within the regulatory structure of the FAA, but also capable of carrying the additional weight of the organ, cameras, and organ tracking, communications and safety systems over an urban, densely populated area—for a longer distance and with more endurance," said Matthew Scassero, MPA, director of UMD's UAS Test Site, part of the A. James Clark School of Engineering. "There's a tremendous amount of pressure knowing there's a person waiting for that organ, but it's also a special privilege to be a part of this critical mission."
In a commentary published in the American Journal of Kidney Diseases, public health researchers suggest adjustments to recently proposed rule changes on how Medicare pays for dialysis services.
Medicare spends approximately $35 billion annually on care for beneficiaries with end-stage renal disease, or kidney failure. That's more than 7 percent of Medicare's total paid claims. Over half a million people receive regular dialysis treatments to manage this condition, with treatment costs averaging about $85,000 a year, according to the study.
IMPACT
Rule changes were proposed about a year ago that would limit the number of dialysis treatments per week that would be paid for by Medicare. Interest groups including nephrologists and patients themselves were concerned that this would limit patient access to innovative treatment options, such as frequent hemodialysis.
Under the current system, Medicare covers three hemodialysis treatments weekly per patient, but it will often pay for additional treatments when the treating nephrologist provides sufficient medical justification.
The recently proposed rule changes would limit such additional payments to exceptional circumstances -- for example, patients with temporary, acute kidney treatment needs. Although nephrologists would not be prevented from providing any "extra" treatments they believe are needed, they would typically bear the costs of doing so.
The researchers discussed the limitations of the current evidence on frequent dialysis treatment, which to date has yielded mixed conclusions. Their suggested changes to Medicare's dialysis payment system were designed to account for these limitations and give Medicare the flexibility to further modify the system in the future as new evidence comes to light.
Under their main proposal, Medicare would establish a new, separate prospective payment system for frequent hemodialysis treatment.
In that way, they said, nephrologists would have greater clarity about how their dialysis care would be paid for, and free them up to pinpoint better ways to treat their patients.
THE TREND
In the first three years of Medicaid expansion due to the Affordable Care Act, the number of patients with end-stage kidney disease who died within a year of starting dialysis decreased in states that expanded Medicaid, compared to non-expansion states, found research published in October.
The adjusted absolute reduction in mortality in expansion states versus non-expansion states was 0.6 percentage points. Since end-stage renal disease affects more than 100,000 Americans each year, 0.6 percentage points equals hundreds of deaths annually.
PKD Research
Select Science
Modeling Kidney Disease with Bioengineered Kidney Organoids

Kidney disease, prevalent in about 14% of the American population, lacks much-needed early interventions to prevent disease progression. Current methods that address kidney disease in a more chronic stage endure additional complexities: for example, artificial kidney devices don’t function as well as a human kidney, and renal transplant procedures need the support of anti-rejection medication.
In this article, we interview Dr. Benjamin Freedman, Assistant Professor at the University of Washington, whose goal is to understand kidney disease in its early stages. “In the medical practice, there's a lot of focus on the end stages of kidney disease and on managing that chronic disease. We are more interested in the early stages and all the different things that can go wrong early on,” says Freedman.
A pioneer in the field of kidney organoids, Freedman chose the 3D cell culture system to study kidney disease. “Organoids are great for this because they can show signs of disease,” Freedman explains. “We can then intervene by treating them with any compound that we're interested in.” A robust model for mimicking disease in vitro, kidney organoids didn’t even exist until a few years ago.
Freedman was the first scientist in the western hemisphere to generate a kidney organoid from pluripotent stem cells. “That was a very exciting moment,” Freedman recalls. “As soon as I saw these structures, I knew that there was something interesting about them. I had all the tools at my disposal to test whether they were indeed kidney.”
And they were.
Bioengineering kidney organoids
Developing and maintaining kidney organoids requires bioengineering capabilities. While the organoids grow and self-assemble from stem cells, their in vitro development deprives them of the vascular perfusion otherwise available in vivo. Tiny microfluidic tubules pass through the kidneys in our bodies, enabling the inlet and outlet of fluid, a resource that kidney organoids grown on a dish don’t receive.
“The kidneys in the body get about 20–25% of the cardiac output at any time point,” explains Freedman. “One thing we're missing in an organoid structure is the ability to perfuse the organoids and the tiny tubules.”
Freedman’s lab has a solution to this problem. “We're trying to make the kidney organoid 2.0, which will incorporate not just the stem cells and their natural ability to form the structures but will also impose a bioengineering design on top of those structures to enable them to really form the very complex types of functional tubules that are found in the body.”

Mimicking in vivo environments
The kidney organoid 2.0 grows on a kidney-on-a-chip device incorporated with extracellular matrix in scaffolded arrangements. By seeding the stem cell-derived kidney cells on this matrix-coated chip, the cells can be grown into controlled shapes and arrangements. “It's a fusion between stem cell and bioengineering fields,” explains Freedman. “The chip, about the size of a credit card, has a tiny tubule structure through which we can perfuse liquid. We can then observe the absorption of solutes such as glucose and ions and see how they're transported from one side of the tubule to the other side.”
The procedure to generate organoids uses a thin layer of Corning® Matrigel® matrix at the bottom of the tissue culture dish. The cells are plated on top of this layer, followed by a second thin layer of Matrigel. “This enables the cells to fold up and create more three-dimensional types of structures which are about 200 microns in diameter,” says Freedman. “These contain all the key lineages of the kidney tissue that we're interested in.”
The Matrigel matrix forms a crucial component of the kidney organoid generation process from pluripotent stem cells. “Matrigel matrix helps the cells to survive. They need a malleable support that they can grow into and form more three-dimensional structures in; something that they can actually change and remodel as they're growing,” Freedman explains.
For the kidney-on-a-chip protocol, the Freedman lab uses a relatively strong and stiff extracellular matrix. “We use Corning Collagen I, a highly concentrated form of collagen because these gels need to be stiff for the cells to take on the right shape that we’re interested in,” says Freedman. “If it’s too soft, the whole thing just falls apart, especially because we have fluid running through it.”
Alternatively, after the organoids have formed, they are placed inside larger Corning Collagen I droplets to stimulate the cells to grow out and populate. “This way, we study the ability of these cells to migrate out of the organoid, which has ramifications for certain disease processes,” says Freedman. “The tubular cells, which are normally sedentary, can actually migrate out when you give them an environment like that.”
Better detached than attached
One of the projects in the Freedman lab involves studying polycystic kidney disease using the kidney organoid model. “In polycystic kidney disease, the tubules, which are normally very narrow, swell up to form large balloon-life structures. They eventually crowd out and destroy all the healthy kidney tissue,” explains Freedman. “We’ve been able to get this to actually happen in our kidney organoids by mutating a gene in the stem cells and derive organoids that are mutants. The organoids swell just like the kidney tubules in the disease.”
This swelling process, however, depends on the microenvironment the organoid finds itself in. Freedman adds: “If you grow the organoids in a condition where they’re essentially floating in media, then it accelerates and exacerbates the disease process.” The lab uses Corning ultra-low attachment plates to grow the organoids detached. “They make these big balloon-like cyst structures very well in the ultra-low attachment plates,” notes Freedman. “They don’t do as well when grown in normal tissue culture conditions.”
The next big thing for 3D cell culture
High-throughput chemical and genetic screening are soon making their way into 3D cell culture biology. “We’re going to move from a phase where we study one drug and its effects at a time to a stage where we’re interrogating the organoids with thousands of compounds,” says Freedman.
“This is something you can’t do in the mammalian animal model, so it’s an exciting time for unbiased discovery using organoids. The big data phase is upon us.”
Kidney Transplant News
From Yahoo Finance
COLLEGE PARK and BALTIMORE, Md., April 26, 2019 /PRNewswire/ -- In a first-ever advancement in human medicine and aviation technology, a University of Maryland (UMD) unmanned aircraft has delivered a donor kidney to surgeons at the University of Maryland Medical Center (UMMC) in Baltimore for successful transplantation into a patient with kidney failure. This successful demonstration illustrates the potential of unmanned aircraft systems (UAS) for providing organ deliveries that, in many cases, could be faster, safer, and more widely available than traditional transport methods.
The momentous flight was a collaboration between aviation and engineering experts at the University of Maryland; transplant physicians and researchers at the University of Maryland School of Medicine (UMSOM) in Baltimore; and collaborators at the Living Legacy Foundation of Maryland.
"This whole thing is amazing. Years ago, this was not something that you would think about," said the kidney recipient, a 44-year-old Baltimore resident who spent eight years on dialysis before undergoing the transplant procedure.The patient was discharged from UMMC on Tuesday.
Maryland faculty and researchers believe this prototype organ transport blazes a trail for the use of UAS to expand access to donated organs, improving outcomes for more people in need of organ transplants.
"This history-making flight not only represents a breakthrough from a technological point of view, but provides an exemplary demonstration of how engineering expertise and ingenuity ultimately serve human needs—in this case, the need to improve the reliability and efficiency of organ delivery to hospitals conducting transplant surgery," said Darryll J. Pines, Ph.D., UMD, dean of the A. James Clark School of Engineering and Nariman Farvardin Professor of Aerospace Engineering. "As astonishing as this breakthrough is from a purely engineering point of view, there's a larger purpose at stake. It's ultimately not about the technology; it's about enhancing human life."
Added Joseph Scalea, MD, assistant professor of surgery at UMSOM, project lead, and one of the surgeons who performed the transplant at UMMC, "As a result of the outstanding collaboration among surgeons, engineers, the Federal Aviation Administration (FAA), organ procurement specialists, pilots, nurses, and, ultimately, the patient, we were able to make a pioneering breakthrough in transplantation."
The many technological firsts of this effort include: a specially designed, high-tech apparatus for maintaining and monitoring a viable human organ; a custom-built UAS with eight rotors and multiple powertrains to ensure consistently reliable performance, even in the case of a possible component failure; the use of a wireless "mesh" network to control the UAS, monitor aircraft status, and provide communications for the ground crew at multiple locations; and aircraft operating systems that combined best practices from both UAS and organ transport standards.
"We had to create a new system that was still within the regulatory structure of the FAA, but also capable of carrying the additional weight of the organ, cameras, and organ tracking, communications and safety systems over an urban, densely populated area—for a longer distance and with more endurance," said Matthew Scassero, MPA, director of UMD's UAS Test Site, part of the A. James Clark School of Engineering. "There's a tremendous amount of pressure knowing there's a person waiting for that organ, but it's also a special privilege to be a part of this critical mission."