Living with PKD
Kidney failure patient tries at-home dialysis

After 42 years of marriage, Roger and Raynelle Cash of Flowery Branch, Georgia, are not just husband and wife.
They are "care partners," working as a team to give Roger the kidney dialysis keeping the 62-year old alive.
Cash's kidneys failed in 2014, after a 20-year long battle with polycystic kidney disease.
"I was in pretty rough shape when I went into dialysis," he says. "So, even in the dialysis, I started feeling better, because they were taking the excess fluid off me."
At the time, Cash would go to a Fresenius Kidney Care dialysis center 3 days a week at 7 am.
There, he would spend up to 4 hours hooked to a machine filtering the toxins from his blood.
It was grueling.
"You feel pretty drained the days you have dialysis," he says. "And, in-center dialysis has a lot more variation, you feel good, feel real bad, feel good, feel real bad."
After his sessions, Cash would go to his job as a maintenance technician at a manufacturing plant.
But instead of feeling better, he started feeling more rundown.
"The longer I stayed on the in-center, daytime dialysis, the worst I felt," he says. "The weaker I got, the worse I felt."
So, Cash switched to an overnight, or nocturnal, dialysis center, thinking he could sleep through 8-hour sessions.
It didn't work.
"I was not sleeping in the center," he says. "I kept trying to work while I was nocturnal. Ultimately, it basically put me into a physical collapse."
He went on medical leave from his job and grew depressed.
That's when Fresenius Kidney Care offered the Cashes another option.
The company would train Roger and Raynelle to do his dialysis at home, using a machine called a dialyzer.
At first, his nephrologist at the time, Dr. Dinesh Chatoth, who is now the Associate Chief Medical Officer at Fresenius, says Cash was reluctant.
"His concerns were, number one, the machine looks pretty complicated," Chatoth remembers. "And, number two, how am I going to use these needles and stick myself in order to go on dialysis?"
Cash had a painful experience as a young boy when he says a nurse mistakenly gave him a penicillin shot in his arm.
It left him uncomfortable with needles, especially the idea of sticking himself.
But, he decided to try home hemodialysis.
"In all honesty I got desperate," Cash says. "Because I wanted to keep working,"
The Cashes went through 2 and half months of training at Fresenius, learning to set up, clean, and sterilize the machine.
They practiced sticking Roger with the needle.
When the time came for the real thing, Raynelle stuck Roger first, then he took over.
"It turned out to be quite a lot easier than I thought it would be."
Dr. Chatoth says the needles can be challenging for patients.
"There is phobia around that," Chatoth says. "But, once we can break through those barriers, once they experience better health, they tend to thrive and do better on dialysis at home."
Chatoth says Medicare and many insurance providers will pay for at-home dialysis for qualified patients.
He says it's less expensive to do the treatment at home, rather than in a center.
About 12 percent of Frensenius Kidney Care patients do at-home dialysis, Chatoth says.
Most, 10 percent do peritoneal dialysis, through a catheter in their belly.
Only 2 percent use the dialyzer for home hemodialysis, like Cash.
Cash sets his own hemodialysis schedule, 4 nights a week, 3 hours at a time.
He does it after work and before going to bed, in case he feels tired.
"Just about 2 and a half months into it, I woke up and said, 'You know, I feel a lot better,'" Cash says. "And, I felt better days and days in a row."
He and Raynelle have now been using the dialyzer for about 3 years.
Roger Cash has had a few bumps in the road, including a recent bout with colon cancer.
But, he says he's feeling good, and back at work.
"I am not as fast as I used to be, and I'm not as strong as I used to be," Cash says. "Don't have the stamina I used to have, but I can still get the job done. That meant a lot to me."
From Renal and Urology News, by Natasha Persaud
Potassium regulation appears to differ in patients with polycystic kidney disease (PKD) compared with other etiologies of chronic kidney disease (CKD), according to the authors of a new study.
In an analysis of 1788 patients from the KNOW-CKD study (KoreaN cohort study for Outcome in patients With Chronic Kidney Disease), patients with PKD had significantly lower serum potassium levels and lower risks for hyperkalemia than other CKD etiologies during CKD stages 1 to 3b. Hyperkalemia prevalence was also lower among the 293 PKD patients than the other CKD groups up to CKD stage 3 to 4. In adjusted analyses, the risk for hyperkalemia was 5 times higher with diabetic nephropathy, 3 times higher with hypertensive nephrosclerosis, and twice as high with glomerulonephritis, than with PKD.
“Taking these results together, it can be suggested that patients with PKD may have a lower risk of hyperkalemia than those with other etiologies of CKD,” Hyoungnae Kim, MD, of Yonsei University in Seoul, and colleagues stated in BMC Nephrology. They added that further use of dual renin-angiotensin-aldosterone system (RAAS) inhibition may be beneficial in decreasing high intrarenal RAAS activity with a low risk of hyperkalemia in patients with PKD.
The team investigated renal potassium handling by measuring urinary angiotensinogen (AGT), a marker of intrarenal RAAS activity. In multivariable linear regression analysis, having a higher urinary AGT to creatinine (Cr) ratio correlated with lower serum potassium. The ratio appeared significantly higher in PKD patients than in other CKD patients. PKD patients also had a significantly higher transtubular potassium gradient (of urinary to serum potassium) that correlated with the AGT to Cr ratio. In PKD patients, a high urinary AGT to Cr ratio was associated with significantly increased risks for kidney function decline and all-cause mortality by 29%.
“In our study, we also showed that urinary AGT/Cr ratio was correlated with decline in renal function and mortality in patients with PKD. To our knowledge, this is the first longitudinal study that has shown urinary AGT as a prognostic marker in patients with PKD,” Dr Kim and his collaborators stated. The researchers acknowledged that the urinary AGT to Cr ratio can be increased with proteinuria, so additional studies are warranted.
PKD Research
From GEN, Genetic Engineering & Biotechnology News, By MaryAnn Labant
Gene therapy, like a car with mechanical problems, has a history of jerking to life and then quickly stalling. Fortunately, gene therapy has the benefit of a kind of roadside assistance, one that comes in the form of gene editing technology, which is becoming more precise. It can help gene therapy run more smoothly. For example, gene editing can now be used to silence or repair a faulty gene, rather than insert an entire gene into the genome. (If a replacement gene is poorly placed, it can cause insertional mutagenesis.) Several forms of high-precision gene editing can give gene therapy a jump, but the most electrifying form is probably CRISPR.
CRISPR is relatively affordable and easy to use. Consequently, it is being embraced by researchers, who are enthusiastically tinkering with CRISPR system components and developing CRISPR systems that are more efficient—and versatile.
Besides dissecting normal and pathogenic pathways, uncovering biomarkers, and identifying drug targets, CRISPR is starting to translate into the clinic. In February 2019, CRISPR Therapeutics and Vertex Pharmaceuticals achieved a major landmark by announcing the first dosing of a patient with a CRISPR-Cas9 therapeutic in a Phase I/II trial. The therapeutic, CTX001, is being used to treat patients with β-thalassemia. Later this year, the trial will be extended to include patients with sickle-cell anemia.
Other CRISPR biotech companies, notably Editas Medicine and Intellia Therapeutics, are also entering the clinic. These companies, which are focused on gene editing therapies for diseases of the eye and liver, share with Sangamo Therapeutics, Cellectis, and Bluebird Bio an interest in developing earlier forms of gene editing technology. These companies are relying on tools such as zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and meganuclease–TAL effector fusions (megaTALs).
Although the older forms of gene editing technology are still relevant, the relative ease of use and affordability of CRISPR-Cas9 suggests that CRISPR will swiftly prove the most abundant platform for gene therapy. Investigators at Exonics Therapeutics recently reported very promising preclinical data using CRISPR gene editing for treating a canine model of Duchenne muscular dystrophy, in which genetic correction of the dystrophin gene appeared to restore limb function in affected King Charles spaniels, supporting advancement to the clinic.
Not only is CRISPR restarting gene therapy, it is also establishing a virtuous cycle of technological development. CRISPR has advantages that recommend it to a growing user base, which is working to enhance CRISPR strategies, methodologies, and nucleases, which keep broadening CRISPR’s appeal, and so on. This cycle may ultimately bring the benefits of gene therapy to patients, including patients suffering multigene disorders.
Mechanistic insights into kidney disease
The kidney is particularly challenging to study. The early stages of kidney disease are difficult to see, and patients with advanced stages of kidney disease often have secondary complications that affect disease understanding.
“There are many different forms of kidney disease, and there is no magic bullet to cure all of them,” says Benjamin “Beno” Freedman, PhD, assistant professor in the Department of Medicine at the University of Washington. “This is where precision medicine helps us to understand the root causes of disease and to intervene early, before dialysis or a transplant is required. Dialysis is crude compared to a functional organ and typically is not a long-term solution, and there is a lack of transplant organs.”
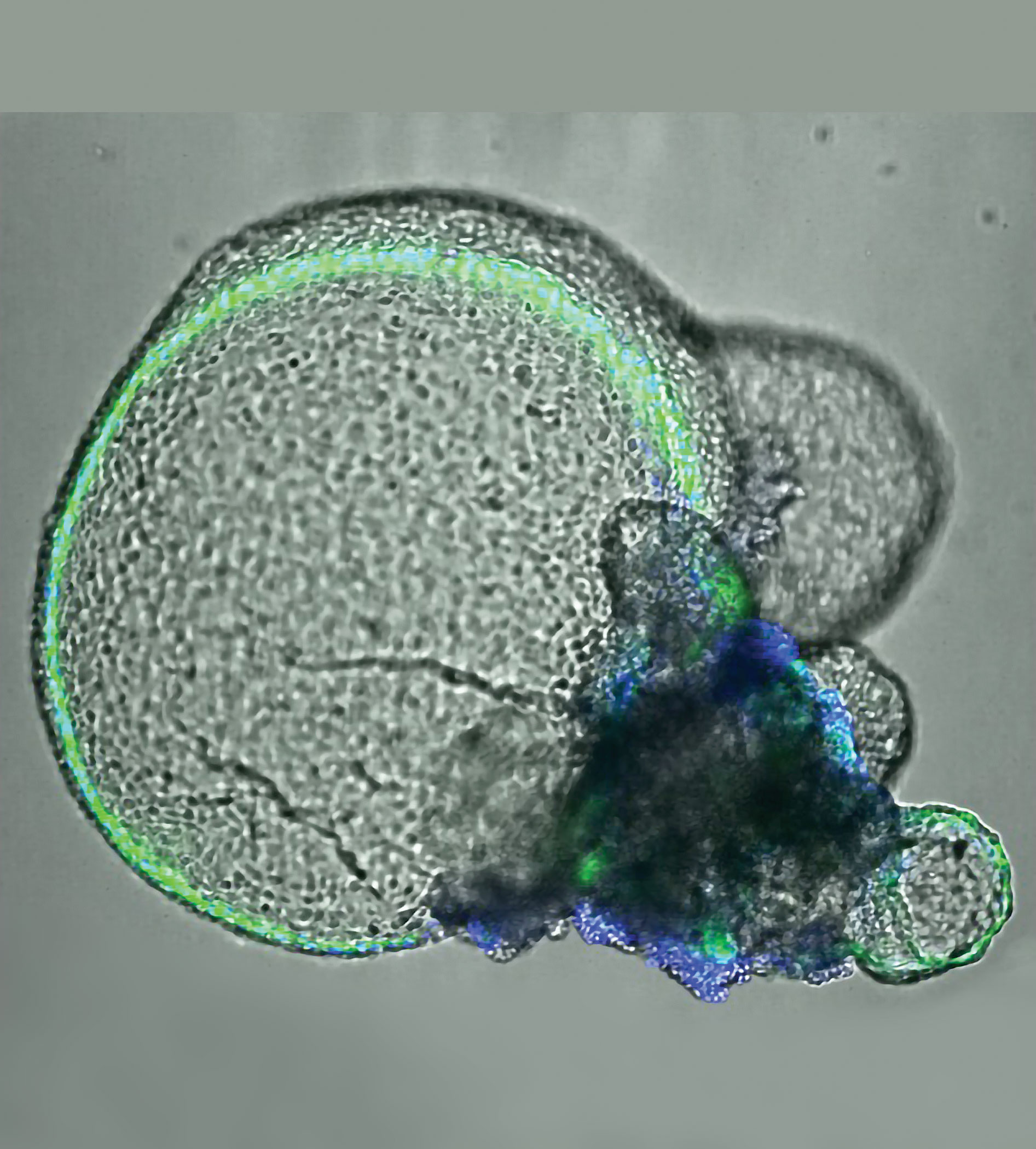
An experimental system must recapitulate some of the kidney’s complex composition and functions. The Freedman laboratory helped to develop the 3D kidney organoid technology that fulfills this need. Kidney organoids are made from stem cells; multiple cell types develop in a relatively intricate nephron-like arrangement that looks similar to the types of structures seen in the kidneys.
The geometry can differ depending on growth conditions, but all kidney organoids are essentially the same regardless of the laboratory of origin. Results are reproducible from laboratory to laboratory, lending standardization and credibility. Using single-cell RNA sequencing and other tools, researchers have found that organoids naturally make about 15 of the approximately 30 types of cells within the kidney.
“One of the very powerful aspects of CRISPR is that it is a functional tool,” Freedman emphasizes. “It changes an outcome and allows us to manipulate these organoids. This makes it possible to start interrogating and identifying the pathways that are involved in constructing the kidney in a functional way and to recreate disease in the organoids.”
Although there is a contribution of genetics to any form of kidney disease, 10–15% of kidney disease is caused by single-gene mutations. If these mutations could be corrected, a big dent would be made in the number of transplants needed. But CRISPR, while more efficient than other technologies, is still not 100% precise—off-target effects remain a concern to many investigators.
Polycystic kidney disease (PKD) is one of the most common genetic diseases, affecting 1 in 600 people. PKD has few treatments and no cure, making it a good candidate for a gene therapy approach. The genes that cause the disease are known, but it is not known how they normally work in the body.
Using the organoids and CRISPR, the Freedman laboratory has developed a PKD disease-in-a-dish model. The model is shedding light on how PKD occurs at the mechanistic level, leading toward finding interventions to halt disease progression.1
“We have also used CRISPR to produce kidney organoids with fluorescent proteins to better visualize how kidney cells work and the effects of treatments on these cells,” Freedman points out. “CRISPR is an excellent research tool because it allows us to investigate genetic pathways in a way that is far more specific than we were able to do before.”
An approach for monogenic blood disorders
Approximately 75% of all monogenic mutations are caused by point mutations—missense, nonsense, and frameshift mutations. Ideally, these disease genes should be corrected directly at their endogenous loci.
Homology-directed repair (HDR) has low repair efficiency. The dominant repair pathway in cells is usually non-homologous end joining (NHEJ). In theory, this repair mechanism should restore the open reading frame (ORF) that is disrupted by a particular disease mutation in approximately one third of the indels. NHEJ should lead to a significant number of ORF reconstitutions.
“My research results show a gene repair efficiency of up to 25% for some CYBB [cytochrome b-245 beta chain] mutations and an on-target mutation rate of 75% at the endogenous CYBB locus,” says Duran Sürün, PhD, a postdoc in medical systems biology at the Dresden University of Technology. “I am convinced that a donor-template-free, RNA-guided Cas9 endonuclease (RGN) approach has a high potential for personalized gene therapy of chronic granulomatous disease (CGD) and other monogenic blood disorders.”2
Although HDR can be utilized for its precise gene repair mechanism, the efficiency is low and requires a positive selection to enrich for gene-corrected cells. This low efficiency arises from double-strand break (DSB) repair in mammalian cells predominately occurring by NHEJ. More importantly, NHEJ represents the dominant DSB repair pathway in hematopoietic stem and progenitor cells (HSPCs).
Furthermore, the donor template required for HDR introduces a risk of random integration. For that reason, the donor-template-free RGN approach is a better strategy for the potential use in personalized gene therapy, Sürün believes.
Currently, the delivery of Cas9 into stem cells proves to be the greatest challenge. Ideally, RGN delivery for gene therapy should be transient and virus free to avoid insertional mutagenesis and immunological side effects. Special consideration should be given to alternative RGN delivery strategies for future experiments.
Advanced electroporation strategies are most promising. RGNs can be delivered as preassembled gRNA/Cas9 protein complexes. Next steps are to test this strategy in patient-specific HSPCs that will be transplanted after ex vivo gene repair into immunodeficient NSG mice.
Genome-editing tools open a wide range of new possibilities in gene manipulation including target-specific gene repair. The results of the first gene therapy studies in patients will be decisive for the further usage of the CRISPR-Cas9 system. According to Sürün, the new Cas9 protein versions hold great promise because they show less off-target activity in cells.
Merging expression levels with safety
CRISPR-Cas9 is a transformative tool that will continue to operate at the leading edge of basic and translational research. According to Mark J. Osborn, PhD, assistant professor, Department of Pediatrics at the University of Minnesota, a recent study3 had the goal of merging the expression levels achieved with lentiviral transgenesis with the safety of gene editing.
When a viral vector is used, it is common for multiple copies of the vector to deliver their payloads to individual cells. If these payloads include strong transcriptional elements, they can promote high-level, sustained gene expression. These elements, whether they are delivered by retro- or lentiviral vectors, often integrate into the genome with a bias for transcriptionally active regions, causing serious adverse events. In contrast, gene editing can result in precision targeting; however, the endogenous promoter may be comparatively weak, resulting in low-level gene expression.
The Minnesota team inserted a powerful transcriptional element upstream of an endogenous start codon to drive high levels of gene expression with defined integration via HDR. Any nuclease has the potential for off-target effects due to overlapping sequence homology at other genomic loci. To reduce the potential off-target effects, Osborn and colleagues decided to rely on proper targeting to drive expression of a selectable marker that aided in selecting for proper targeting events.
Osborn indicates that his team is further assessing its strategy by defining and mapping potential off-target sites. The hope is that the approach will streamline engineering and make high-level gene expression safer than when integrating vectors are employed.
Identifying off-target effects
Identifying unwanted off-target effects remains an essential requirement for clinical translation of genome editing. Unfortunately, a well-validated method that can reliably identify these events in vivo has been lacking.
Filling this void is a sensitive, unbiased, and generalizable strategy called VIVO (Verification of In Vivo Off-targets). Developed at the Massachusetts General Hospital laboratory of J. Keith Joung, MD, PhD, VIVO allows for the robust identification of genome-wide CRISPR-Cas off-target effects in vivo. VIVO was described in a 2018 Naturearticle,4 which demonstrated that CRISPR-Cas can induce substantial off-target mutations in vivo and that appropriately designed guide RNAs can direct efficient in vivo editing without inducing detectable off-target mutations.
The VIVO approach can be used irrespective of the delivery method for CRISPR-Cas. It is envisioned as a two-step strategy: The first in vitro step uses the CIRCLE-seq method to identify potential off-target cleavage sites of a nuclease of interest on purified genomic DNA.5 Next, off-target sites identified by CIRCLE-seq are examined for evidence of indel mutations in the genomic DNA of target tissues in vivo that have been treated with the nuclease.
The in vivo detection limit of VIVO is limited by the current error rate of sequencing. Previous in vivo studies used computational in silico approaches or the GUIDE-seq method performed on surrogate cells in culture to identify off-target effects.
The results presented by Joung’s group provided the first convincing demonstration that CRISPR-Cas can induce significant off-target mutations in vivo, and, importantly, that these off-target effects can be eliminated with appropriately designed guide RNAs.
No comments:
Post a Comment