From MDMag, by Kenny Walter
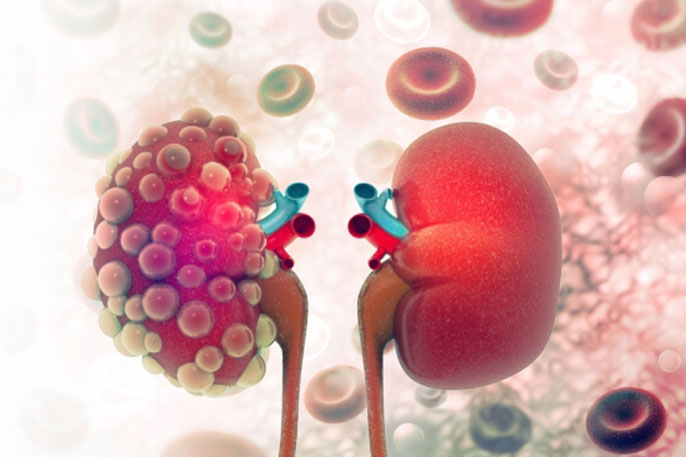
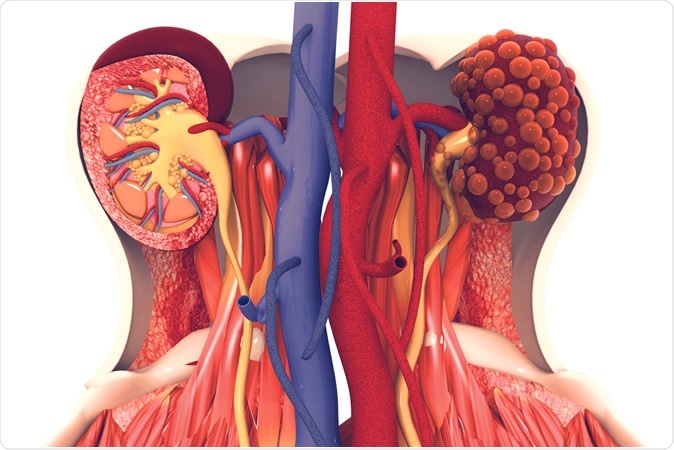
Octreotide-LAR treatment could be beneficial to patients suffering from autosomal dominant polycystic kidney disease (ADPKD).
In a poster presented at the American Society of Nephrology (ASN) Kidney Week in Washington, D.C., investigators, led by Satyanarayana Vaidya, MD, Emory School of Medicine, evaluated the efficacy of Octreotide-LAR on disease progression based on a meta-analysis of published literature across all stages of chronic kidney disease due to ADPKD.
The team identified all placebo-controlled randomized trials of Octreotide-LAR through a literature search and analyzed the efficacy—rate of cyst growth and kidney function decline—as well as safety outcomes.
Ultimately, they found 4 trials that fulfilled the requirements, with a total of 445 patients.
The results showed were positive for Octreotide-LAR.
“Compared to placebo, Octreotide-LAR showed a significant reduction of total kidney volume, standard mean difference -.41[ 95% CI, -.69--0.12], P =.005 but a comparable mean reduction in glomerular filtration rate standard mean difference .01 [95% CI, -.17-.20], P =.90 and rate of adverse events RR, 1.46 [0.82-2.61], P =.20,” the authors wrote.
Participants in the trial were adults with a clinical and ultrasound diagnosis of ADPKD with glomerular filtration rate of ≥15 ml/min/1.73 m2, with the exclusion of diabetics and patients with poorly controlled hypertension (BP> 180/110 mmHg).
The outcomes included mean total kidney volume and decrease in glomerular filtration rate, compared using a standard mean difference.
ADPKD is the most common hereditary kidney disease, characterized by tubular epithelial cell proliferation (ECP) and fluid secretion leading to cystic kidney enlargement and progressive renal failure in the majority of cases.
The cyclic adenosine monophosphate (cAMP) pathway has been found in the past in both epithelial cell proliferation and fluid secretion.
Somatostatin and its synthetic analogues have shown in the past to inhibit invitro adenyl cyclase activity and slow cyst growth in both underpowered studies in either early or more advanced stages of ADPKD.
However, the study could yield better outcomes for patients with ADPKD.
“Octreotide-LAR delays the cyst growth across all stages of kidney disease in ADPKD, without a clear beneficial effect on kidney function, or a significant difference in adverse outcomes,” the authors wrote. “Longer follow-up is required to elucidate a potential beneficial role of Octreotide-LAR on kidney function.”
Kidney Transplant
From Time, BY LISA EMMOTT
2017 ended as a banner year for my family, but things didn’t look great at the start. A death sentence met us in a boxing ring, and we had to school ourselves on fighting to live. I never thought much about the 37 million American adults who suffer from kidney disease until my husband Neil became one of them.
Celebrating our first year of marriage in 2001, we learned by accident through an unrelated medical exam that my husband has polycystic kidney disease, an illness which causes the kidneys to fill with cysts over time, rendering the organs unable to function properly. There is no cure. There was nothing to do but wait for my husband’s kidney function to decline below 20%, the point at which either dialysis or a transplant would be considered to prolong life.
It would be 16 more years before Neil would enter end-stage renal failure, the final, permanent phase of chronic kidney disease where the organs no longer function, and in early 2017, he joined the waitlist for a transplant, alongside some 100,000 others in the U.S. Those on the waitlist face a three-to-10 year wait for a deceased donor kidney, and the statistics are grim. Kidney disease is the ninth most common cause of death in the U.S., and while Medicare covers the cost of dialysis for kidney failure, it is an exhausting treatment process with low survival rates. America’s kidney shortage kills 43,000 people per year, as those on the waitlist sit hoping for “the call” that an organ has become available.
Much hope was given to kidney disease patients in July when the federal government announced an executive order aimed at overhauling the care of kidney disease. The move aims to reduce the number of kidney failures by early monitoring and preventative care, and to make more kidneys available for transplant by examining the rate of discarded kidneys from deceased donors. The administration also plans to address disincentives to living donation such as financial hardship during time off work.
While this call to action is long overdue, continued education is needed to empower patients to navigate the transplant process. We were bombarded with a plethora of medical information during my husband’s extensive transplant evaluation, but we received no guidance about his best shot at a long and healthy life: a kidney from a living donor. Determined not to watch my husband deteriorate on the deceased donor waitlist, I went to work finding a living kidney donor, a challenging job that typically falls on the patient or family members. I quickly learned that having a willing donor is a far cry from having an approved donor.
Living-donor criteria are very stringent in order to protect the donor’s long-term health. While each transplant center has its own specific criteria, being overweight, having uncontrolled hypertension, diabetes, cancer, or a serious mental health condition can all rule a person out from donating a kidney. During my medical evaluation, I was diagnosed with fibromuscular dysplasia, a progressive twisting and beading of the renal arteries. All the other willing potential donors in our family were also deemed medically ineligible. With this devastating news, we went public with our search for a donor. I spoke about my husband’s plight at local community groups, we printed signs and cards to post in local businesses, and campaigned on social media. Eventually, a number of people came forward to donate and in the end, two teachers at our daughter’s school were approved.
From MedicalXpress, University of California, San Francisco
The Kidney Project, a national effort to develop an implantable bio-artificial kidney that could eliminate the need for dialysis, will announce a key milestone in a November 7, 2019 presentation at the American Society of Nephrology Kidney Week 2019 conference in Washington, DC.
The team will report that UC San Francisco scientists have successfully implanted a prototype kidney bioreactor containing functional human kidney cells into pigs without significant safety concerns. The device, which is about the size of a deck of cards, did not trigger an immune reaction or cause blood clots in the animals, an important milestone on the road to future human trials.
"This is the first demonstration that kidney cells can be implanted successfully in a large animal without immunosuppression and remain healthy enough to perform their function. This is a key milestone for us," said Kidney Project co-lead Shuvo Roy, Ph.D., a faculty member in the Department of Bioengineering and Therapeutic Sciences, a joint department of the UCSF Schools of Pharmacy and Medicine. "Based on these results, we can now focus on scaling up the bioreactor and combining it with the blood filtration component of the artificial kidney."
UCSF-Vanderbilt Kidney Project Aims to Eliminate Dialysis
Nearly 750,000 Americans—and two million people around the world—are treated for end-stage renal disease (ESRD), and rates of kidney disease are growing rapidly, leading to an urgent shortage of kidneys for transplant. As of 2016 there were only 21,000 donor kidneys available for transplant in the U.S. on a waiting list of nearly 100,000 and extending five to ten years.
Most patients awaiting a kidney transplant survive by undergoing long and cumbersome dialysis treatments multiple times a week to clear toxins from their blood, but dialysis does not replace many essential kidney functions and on average, only 35 percent of dialysis patients remain alive after five years. Dialysis and other treatments for ESRD, which are universally covered by Medicare, cost $35 billion in 2016, representing seven percent of Medicare's annual budget.
The Kidney Project [pharm.ucsf.edu/kidney] is led by Roy and Vanderbilt University Medical Center nephrologist William H. Fissell, MD, who for more than a decade have been working to develop an implantable bio-artificial kidney with the goal of eliminating dialysis and easing the shortage of donor kidneys.
The implantable device being developed by The Kidney Project consists of two components: an blood filtration system called the hemofilter, which removes toxins from the blood by passing it through silicon membranes fabricated with precisely shaped nanometer-scale pores; and a bioreactor, which contains cultured human kidney cells intended to perform other kidney functions, such as maintaining adequate fluid volume and blood pressure, adjusting salt levels, and producing essential hormones.
Following promising studies in large animals, The Kidney Project's hemofiltration system is currently awaiting FDA approval for an initial clinical trial to evaluate its safety. The bioreactor technology has been tested in laboratory experiments but so far had not been implanted into animals.
Bioreactor Containing Human Kidney Cells Implanted in Pigs Without Immune Reaction or Blood Clots
In The Kidney Project's November 7 Kidney Week presentation, Rebecca Gologorsky, MD, a UCSF Surgical Innovations Fellow on the team, will show how silicon membranes inside the implanted bioreactor protect the enclosed human kidney cells from the host immune system by keeping blood-borne immune cells and proteins out of the device.
"It has been a holy grail of transplant therapies to find ways to avoid the need for lifelong immunosuppressive drugs that are often required to prevent immune rejection," Roy said. "These drugs not only expose patients to infection and other harmful side-effects but have been shown to directly harm transplanted cells and organs, eroding the therapeutic benefit of transplants over time."
Another key benefit of avoiding immunosuppression is its cost to patients, Roy says: "Medicare currently covers dialysis for life, but immunosuppressive drugs are covered for just the first three years following transplant. Many patients who receive kidney transplants ultimately lose the new organ because they weren't able to afford the immunosuppressive drugs needed to keep it healthy."
Roy's team also carefully engineered the prototype bioreactor to avoid triggering blood clots that could lead to pulmonary embolism or stroke, a major challenge faced by all patients with long-term medical implants. They achieved this by coating the silicon membrane filters that contact the blood with biologically friendly molecules and engineering the device to avoid the turbulent blood flow that can also trigger clotting.
"We couldn't use the standard blood-friendly coatings that have been developed for heart valves, catheters, and other devices because they are so thick that they would completely block the pores of our silicon membranes," Roy said. "One of our accomplishments has been to engineer a suitable surface chemistry on our silicon membranes that makes them look biologically friendly to blood."
The results, Roy says, demonstrate progress towards The Kidney Project's hoped-for clinical "trifecta": a heart-powered device that runs without batteries or other external connections that could introduce infection risk, and which can clean the blood without anti-rejection drugs or blood thinners.
The researchers now aim to scale up the prototype bioreactor to contain more cells in order to test whether the implanted device can supplement kidney function in animals with kidney failure, with the ultimate goal of eventually moving the device to human safety trials.
"Advancing a complex cell therapy like this into the clinic will not be a trivial task—for instance, it will require substantial investments in cell production and characterization in controlled GMP facilities to avoid any possibility of contamination," Roy said. "Now we've confirmed that we're on the right track to move forward with these efforts."
The team will report that UC San Francisco scientists have successfully implanted a prototype kidney bioreactor containing functional human kidney cells into pigs without significant safety concerns. The device, which is about the size of a deck of cards, did not trigger an immune reaction or cause blood clots in the animals, an important milestone on the road to future human trials.
"This is the first demonstration that kidney cells can be implanted successfully in a large animal without immunosuppression and remain healthy enough to perform their function. This is a key milestone for us," said Kidney Project co-lead Shuvo Roy, Ph.D., a faculty member in the Department of Bioengineering and Therapeutic Sciences, a joint department of the UCSF Schools of Pharmacy and Medicine. "Based on these results, we can now focus on scaling up the bioreactor and combining it with the blood filtration component of the artificial kidney."
UCSF-Vanderbilt Kidney Project Aims to Eliminate Dialysis
Nearly 750,000 Americans—and two million people around the world—are treated for end-stage renal disease (ESRD), and rates of kidney disease are growing rapidly, leading to an urgent shortage of kidneys for transplant. As of 2016 there were only 21,000 donor kidneys available for transplant in the U.S. on a waiting list of nearly 100,000 and extending five to ten years.
Most patients awaiting a kidney transplant survive by undergoing long and cumbersome dialysis treatments multiple times a week to clear toxins from their blood, but dialysis does not replace many essential kidney functions and on average, only 35 percent of dialysis patients remain alive after five years. Dialysis and other treatments for ESRD, which are universally covered by Medicare, cost $35 billion in 2016, representing seven percent of Medicare's annual budget.
The Kidney Project [pharm.ucsf.edu/kidney] is led by Roy and Vanderbilt University Medical Center nephrologist William H. Fissell, MD, who for more than a decade have been working to develop an implantable bio-artificial kidney with the goal of eliminating dialysis and easing the shortage of donor kidneys.
The implantable device being developed by The Kidney Project consists of two components: an blood filtration system called the hemofilter, which removes toxins from the blood by passing it through silicon membranes fabricated with precisely shaped nanometer-scale pores; and a bioreactor, which contains cultured human kidney cells intended to perform other kidney functions, such as maintaining adequate fluid volume and blood pressure, adjusting salt levels, and producing essential hormones.
Following promising studies in large animals, The Kidney Project's hemofiltration system is currently awaiting FDA approval for an initial clinical trial to evaluate its safety. The bioreactor technology has been tested in laboratory experiments but so far had not been implanted into animals.
Bioreactor Containing Human Kidney Cells Implanted in Pigs Without Immune Reaction or Blood Clots
In The Kidney Project's November 7 Kidney Week presentation, Rebecca Gologorsky, MD, a UCSF Surgical Innovations Fellow on the team, will show how silicon membranes inside the implanted bioreactor protect the enclosed human kidney cells from the host immune system by keeping blood-borne immune cells and proteins out of the device.
"It has been a holy grail of transplant therapies to find ways to avoid the need for lifelong immunosuppressive drugs that are often required to prevent immune rejection," Roy said. "These drugs not only expose patients to infection and other harmful side-effects but have been shown to directly harm transplanted cells and organs, eroding the therapeutic benefit of transplants over time."
Another key benefit of avoiding immunosuppression is its cost to patients, Roy says: "Medicare currently covers dialysis for life, but immunosuppressive drugs are covered for just the first three years following transplant. Many patients who receive kidney transplants ultimately lose the new organ because they weren't able to afford the immunosuppressive drugs needed to keep it healthy."
Roy's team also carefully engineered the prototype bioreactor to avoid triggering blood clots that could lead to pulmonary embolism or stroke, a major challenge faced by all patients with long-term medical implants. They achieved this by coating the silicon membrane filters that contact the blood with biologically friendly molecules and engineering the device to avoid the turbulent blood flow that can also trigger clotting.
"We couldn't use the standard blood-friendly coatings that have been developed for heart valves, catheters, and other devices because they are so thick that they would completely block the pores of our silicon membranes," Roy said. "One of our accomplishments has been to engineer a suitable surface chemistry on our silicon membranes that makes them look biologically friendly to blood."
The results, Roy says, demonstrate progress towards The Kidney Project's hoped-for clinical "trifecta": a heart-powered device that runs without batteries or other external connections that could introduce infection risk, and which can clean the blood without anti-rejection drugs or blood thinners.
The researchers now aim to scale up the prototype bioreactor to contain more cells in order to test whether the implanted device can supplement kidney function in animals with kidney failure, with the ultimate goal of eventually moving the device to human safety trials.
"Advancing a complex cell therapy like this into the clinic will not be a trivial task—for instance, it will require substantial investments in cell production and characterization in controlled GMP facilities to avoid any possibility of contamination," Roy said. "Now we've confirmed that we're on the right track to move forward with these efforts."
Organ-on-a-chip models could fundamentally change the way drugs make their journey to clinical trials and reduce the need for animals in laboratory experiments. The new testing technology is of great interest to pharmaceutical companies looking to better understand the effects of medicines—especially the unintended consequences of their administration—and will facilitate study of not only toxicity, but also the mechanism by which drugs work and the timing of their delivery and physiological response, according to Vanderbilt University professor John Wikswo, a biological physicist and founding director of the Vanderbilt Institute for Integrative Biosystems Research and Education.
As Wikswo defines them, organs-on-chips are microfluidic devices that are populated with living cells, most often human, to create two-dimensional (2D) or three-dimensional (3D) microphysiological systems that recapitulate human physiology better than cells grown on flat plastic. They’re in the same class with, but more complex than, either spheroids (typically formed from cancer cell lines or tumor biopsies) and organoids (self-organized, organ-specific cultures typically derived from stem cells). The coupling together of different organ-on-chip models allows researchers to mimic human physiology better than any single chip could do individually.
The human body has roughly 200 organs and they don’t yet all have a chip counterpart, says Wikswo. Around two dozen of them have been micro-engineered to date—including the blood-brain barrier, gut, kidney, lung, parts of the female and male reproductive systems, the mammary gland, bone marrow, cardiac and skeletal muscle, and the bone-cartilage interface—as well as multiple liver-on-a-chip models.
Wikswo holds 18 patents and has multiple patents pending related to the instrumentation and control of cells and the support hardware for organs-on-chips. These include the MicroFormulator, an innovative platform for controlling the concentration of drugs in each well of a multiwell plate commonly used in biomedical and clinical research. Organs-on-chips, at their core, are all microfluidic cell culture devices.
The organ chips themselves are transparent and roughly the size of an AA battery, each with its own instrumentation and software. They vary by purpose as well as size, shape and what they grow on, says Wikswo. One of the new economy models coming out of Vanderbilt is shaped like a puck, and others have been engineered to resemble bendy posts or curling levers.
As Wikswo defines them, organs-on-chips are microfluidic devices that are populated with living cells, most often human, to create two-dimensional (2D) or three-dimensional (3D) microphysiological systems that recapitulate human physiology better than cells grown on flat plastic. They’re in the same class with, but more complex than, either spheroids (typically formed from cancer cell lines or tumor biopsies) and organoids (self-organized, organ-specific cultures typically derived from stem cells). The coupling together of different organ-on-chip models allows researchers to mimic human physiology better than any single chip could do individually.
The human body has roughly 200 organs and they don’t yet all have a chip counterpart, says Wikswo. Around two dozen of them have been micro-engineered to date—including the blood-brain barrier, gut, kidney, lung, parts of the female and male reproductive systems, the mammary gland, bone marrow, cardiac and skeletal muscle, and the bone-cartilage interface—as well as multiple liver-on-a-chip models.
Wikswo holds 18 patents and has multiple patents pending related to the instrumentation and control of cells and the support hardware for organs-on-chips. These include the MicroFormulator, an innovative platform for controlling the concentration of drugs in each well of a multiwell plate commonly used in biomedical and clinical research. Organs-on-chips, at their core, are all microfluidic cell culture devices.
The organ chips themselves are transparent and roughly the size of an AA battery, each with its own instrumentation and software. They vary by purpose as well as size, shape and what they grow on, says Wikswo. One of the new economy models coming out of Vanderbilt is shaped like a puck, and others have been engineered to resemble bendy posts or curling levers.
Read More.
Russell Desmond received a letter a few weeks ago from the American Kidney Fund that he said felt like “a smack on the face.”
The organization informed Desmond, who has kidney failure and needs dialysis three times a week, that it will no longer help him pay for his private health insurance plan — to the tune of about $800 a month.
“I am depressed about the whole situation,” said the 58-year-old Sacramento resident. “I have no clue what I’m going to do.”
Desmond has Medicare, but it doesn’t cover the entire cost of his care. So, with assistance from the American Kidney Fund, he pays for a private plan to cover the difference.
Now, the fund, which helps about 3,700 Californians pay their premiums and out-of-pocket costs, is threatening to pull out of California because of a new state law that is expected to cut into the dialysis industry’s profits — leaving patients like Desmond scrambling.
The letter portrayed the fund as helpless. “We are heartbroken at this outcome,” it read. “Ending assistance in California is the last thing we want to do.”
But supporters of the new law are calling the threat a scare tactic. State Assemblyman Jim Wood (D- Healdsburg), the author of AB-290, said there is nothing in the measure that prohibits the fund from continuing to provide financial assistance to patients.
“AKF has simply made a conscious decision, without merit, to leave the state despite the many accommodations I made by amending the bill in the Senate to ensure that it can continue to operate in California,” Wood said in a written statement.
What’s behind this dispute is the tight relationship between the American Kidney Fund and the companies that provide dialysis, which filters the blood of people whose kidneys are no longer doing the job.
People on dialysis usually qualify for Medicare, the federal health insurance program for people 65 and older, and those with kidney failure and certain disabilities. If they’re low income, they may also qualify for Medicaid, which is called Medi-Cal in California.
But dialysis companies can get higher reimbursements from private insurers than from public coverage. And one way to keep dialysis patients on private insurance is by giving them financial assistance from the American Kidney Fund, which helps nearly 75,000 low-income dialysis patients across the country.
The fund gets most of its money from DaVita and Fresenius Medical Care, the two largest dialysis companies in the country. The fund does not disclose its donors, but an audit of its finances reveals that 82% of its funding in 2018 — nearly $250 million — came from two companies.
Insurance plans, consumer advocacy groups and unions have accused the American Kidney Fund of helping dialysis providers steer patients into private insurance plans in exchange for donations from the dialysis industry. Wood said his bill is intended to discourage that practice.
American Kidney Fund CEO LaVarne Burton denied the accusations and said her group plays no role in patients’ coverage choices.
Starting in 2022, the new law will limit the private-insurance reimbursement rate that dialysis companies receive for patients who get assistance from groups such as the American Kidney Fund to the rate that Medicare pays. The rate change won’t apply to patients who are currently receiving assistance as long as they keep the same health plans. The bill will also address a similar dynamic in drug treatment programs.
To determine which patients receive financial aid, the law will require third-party groups to disclose patients’ names to health insurers starting July 1, 2020.
Read More
Dialysis
From California HealthLine, By Ana B. Ibarra
Russell Desmond received a letter a few weeks ago from the American Kidney Fund that he said felt like “a smack on the face.”
The organization informed Desmond, who has kidney failure and needs dialysis three times a week, that it will no longer help him pay for his private health insurance plan — to the tune of about $800 a month.
“I am depressed about the whole situation,” said the 58-year-old Sacramento resident. “I have no clue what I’m going to do.”
Desmond has Medicare, but it doesn’t cover the entire cost of his care. So, with assistance from the American Kidney Fund, he pays for a private plan to cover the difference.
Now, the fund, which helps about 3,700 Californians pay their premiums and out-of-pocket costs, is threatening to pull out of California because of a new state law that is expected to cut into the dialysis industry’s profits — leaving patients like Desmond scrambling.
The letter portrayed the fund as helpless. “We are heartbroken at this outcome,” it read. “Ending assistance in California is the last thing we want to do.”
But supporters of the new law are calling the threat a scare tactic. State Assemblyman Jim Wood (D- Healdsburg), the author of AB-290, said there is nothing in the measure that prohibits the fund from continuing to provide financial assistance to patients.
“AKF has simply made a conscious decision, without merit, to leave the state despite the many accommodations I made by amending the bill in the Senate to ensure that it can continue to operate in California,” Wood said in a written statement.
What’s behind this dispute is the tight relationship between the American Kidney Fund and the companies that provide dialysis, which filters the blood of people whose kidneys are no longer doing the job.
People on dialysis usually qualify for Medicare, the federal health insurance program for people 65 and older, and those with kidney failure and certain disabilities. If they’re low income, they may also qualify for Medicaid, which is called Medi-Cal in California.
But dialysis companies can get higher reimbursements from private insurers than from public coverage. And one way to keep dialysis patients on private insurance is by giving them financial assistance from the American Kidney Fund, which helps nearly 75,000 low-income dialysis patients across the country.
The fund gets most of its money from DaVita and Fresenius Medical Care, the two largest dialysis companies in the country. The fund does not disclose its donors, but an audit of its finances reveals that 82% of its funding in 2018 — nearly $250 million — came from two companies.
Insurance plans, consumer advocacy groups and unions have accused the American Kidney Fund of helping dialysis providers steer patients into private insurance plans in exchange for donations from the dialysis industry. Wood said his bill is intended to discourage that practice.
American Kidney Fund CEO LaVarne Burton denied the accusations and said her group plays no role in patients’ coverage choices.
Starting in 2022, the new law will limit the private-insurance reimbursement rate that dialysis companies receive for patients who get assistance from groups such as the American Kidney Fund to the rate that Medicare pays. The rate change won’t apply to patients who are currently receiving assistance as long as they keep the same health plans. The bill will also address a similar dynamic in drug treatment programs.
To determine which patients receive financial aid, the law will require third-party groups to disclose patients’ names to health insurers starting July 1, 2020.
Read More