From Kidney International: Official Journal of the International Society of Nephrology
CD8+ T cells modulate autosomal dominant polycystic kidney disease progression
Autosomal dominant polycystic kidney disease (ADPKD) is the most common, potentially lethal monogenic nephropathy caused predominantly by mutations to either PKD1 or PKD2.1, 2, 3, 4ADPKD accounts for 8% to 10% of patients receiving renal replacement therapy for end-stage renal disease (ESRD) worldwide5 and affects roughly 1:400 to 1:1000 people.6, 7 The disease is characterized by dysregulated growth of renal epithelial cells leading to progressive, bilateral fluid-filled renal cysts and resulting in ESRD in about 50% of patients by middle age.8, 9Extrarenal manifestations, such as liver and pancreatic cysts or cardiovascular abnormalities, further decrease quality of life and increase morbidity and mortality.10, 11 Previous research focused on targeting pathways central to cyst pathology, such as cyclic adenosine monophosphate/protein kinase A, epidermal growth factor, and mammalian target of rapamycin,12 have provided positive data in murine preclinical trials, but their efficacy in humans was modest at best.13, 14, 15, 16, 17 Hence the number of US Food and Drug Administration (FDA)–approved compounds for the treatment of ADPKD are limited, and ESRD is managed by either dialysis or kidney transplant.18 Thus an urgent need exists to explore new treatment options that can slow the progression of ADPKD and prevent advancement to ESRD.
Whereas mutations in PKD1 or PKD2 mediate ADPKD initiation and progression,19, 20 observed intra- and interfamilial phenotypic heterogeneity, ranging from in utero onset21, 22 to adequate renal function at old age,23 exceeds genic effects,3, 24 suggesting that additional, nongenetic factors contribute to disease progression. Further, the functional role of the PKD1 and PKD2proteins, polycystin-1 and polycystin-2, while extensively studied, remains elusive, leaving many open questions regarding the mechanisms that drive cystogenesis.25, 26, 27, 28
Although ADPKD historically has been considered a “neoplasia in disguise,”29 the significant similarities between ADPKD and cancer have been rediscovered more recently.30 In fact, many of the cancer hallmarks as defined by Hanahan and Weinberg31 are applicable to ADPKD (e.g., sustained proliferation,12, 30, 32 genomic instability,33, 34, 35 deregulated cellular energetics,36, 37and inflammation/avoiding immune destruction38, 39, 40, 41, 42, 43, 44, 45, 46, 47). Importantly, interstitial inflammation has been reported in human patients with ADPKD, as well as in animal models of the disease.40 In concordance with an inflammatory response, increased levels of pro-inflammatory cytokines, such as monocyte chemoattractant protein-1 and tumor necrosis factor–α, were detected in cyst fluid of patients with ADPKD, and anti-inflammatory therapies have been shown to attenuate disease progression in animal models.38, 39, 40 Furthermore, macrophage infiltration can be observed in orthologous and nonorthologous ADPKD models at advanced disease stage,41, 42, 43 and a few reports show CD4+ T cells, mast cells, and neutrophils in the interstitium of patients with ADPKD.44, 45, 46 Additionally, historic data showed that murine PKD models raised in germ-free environments present with milder cystic disease,47 suggesting a role for the immune system in PKD. In fact, it was shown that M2-like macrophages can promote cyst growth in murine models of autosomal recessive PKD (ARPKD) and ADPKD and that their depletion slows renal and hepatic cystogenesis.41, 42, 48 However, to date, no research in the literature addresses the role of the adaptive immune system in ADPKD initiation and progression.
Targeting adaptive immunity has become a central focus in developing new therapeutic approaches in multiple malignancies.49, 50 In many cancers, increased numbers of tumor-infiltrating T cells are associated with better prognosis,51 consistent with a role for these cells in inhibiting tumor progression. However, the role of different T-cell subtypes is complex because of their heterogeneity.52 As such, different populations have either pro-tumorigenic (e.g., regulatory T cells [Tregs]) or antitumorigenic (e.g., CD8+ T cell) roles. Additionally, cancers have developed multiple cellular and molecular pathways to suppress T-cell functions. Strategies targeting the interaction of specific T cells with cancer cells have shown recent clinical success, leading to FDA approval of checkpoint inhibitors that target the interactions of programmed cell death protein-1 (PD-1) with its ligand PD-L1, resulting in reactivation of antitumor CD8+ T cells.53, 54 The progress made in the field of cancer research, specifically the function of T cells in tumorigenesis, may yield new ideas and avenues for ADPKD research. Thus, an essential first step forward is to understand the role of T cells in ADPKD progression.
Here, we characterized T-cell subpopulations in an orthologous mouse model of ADPKD that reproduces critical features of the human disease, including a slow rate of progression. We found that T cells increase in correlation with disease severity and localize specifically to cystic lesions. Importantly, our results define a functional role for CD8+ T cells in inhibiting ADPKD progression, highlighting the potential to adapt cancer immunotherapy strategies to ADPKD.
Results
Renal T-cell numbers are increased in the C57Bl/6 Pkd1RC/RC model compared with wild type
The homozygous Pkd1 p.R3277C (Pkd1RC/RC)55 model genetically and physiologically mimics human ADPKD. It harbors a knock-in mutation that mimics a hypomorphic allele identified in ADPKD families56 and presents over time in the C57Bl/6 background with slowly progressive disease with moderate increases in percent kidney weight/body weight (%KW/BW), renal cystic/fibrotic area (index), chronic inflammation (increased renal interleukin-6/decreased interleukin-10 levels), as well as mild renal function decline (Supplementary Figure S1; Supplementary Table S1). To evaluate how the cystic microenvironment (CME), specifically T cells, differ between C57Bl/6 wild type (WT) and Pkd1RC/RC mice and to correlate changes in the adaptive immune system profile with cystogenesis progression, we used flow cytometry analysis of renal single cell suspensions and analyzed mice at 3, 6, and 9 months of age. In Pkd1RC/RCC57Bl/6 mice at 3 and 6 months, when the cystic disease is mild/moderate, respectively, we detected a statistically significant increase in immune cells (CD45+) and T cells (T-cell receptor β+[TCRβ+]) in Pkd1RC/RC compared with WT mice, but the most striking increase occurred at 9 months of age, the investigated time point at which PKD is most severe (Figure 1a and b; Supplementary Figure S1; Supplementary Table S1). The same pattern was observed for both cytotoxic T cells (CD8+) and helper T cells (CD4+, Figure 1c and d; Supplementary Table S1). Importantly, as shown by immunofluorescence, T cells (CD3+, CD4+, CD8+) specifically localized around cystic lesions even at mild stages of cystogenesis (3 months) when the global increase of T-cell number in the kidney was modest (Figure 1e and f; Supplementary Figure S2). At 9 months a more diffuse increase of T cells was notable by immunofluorescence, characterized by increased localization to noncystic areas, likely accounting for the striking increase in T-cell number at 9 months observed by flow cytometry (Figure 1b and e). This finding likely reflects the adaptive immune system’s response to increasing tubular atrophy and interstitial expansion/inflammation that can be observed in 9-month-old Pkd1RC/RC C57Bl/6 mice, despite the slowly progressive/mild disease (Supplementary Figure S1C). Importantly, the increase in T-cell numbers between C57Bl/6 WT and Pkd1RC/RC mice was specific to kidney disease, as no changes were observed in spleens (Supplementary Figure S3). Read More...
Gift of Life
From News24, Cape Town, South Africa
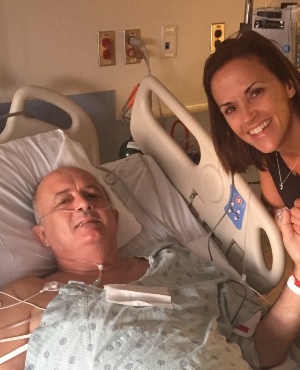
A dad in desperate need of a kidney has been saved by two teachers at his daughter's school – sparking a donor chain reaction that saw eight more lives being saved.
Dad-of-two Neil Emmott was diagnosed with polycystic kidney disease (PKD) – which causes cysts to grow on the kidneys – in 2001, and over the next 15 years his vital organs began to fail.
In 2016 the usually shy and private Neil, from Fort Lauderdale in Florida in the US, was forced to make his plight public as his search for a suitable kidney became more desperate.
By the beginning of 2017, the 56-year-old’s kidney functionality was down to just 11%, leaving a single percentage of function before he’d need to be put on emergency dialysis.
When Neil’s wife, Lisa (44), and his brother, Gordon, were ruled out as potential donors because of minor health issues, Lisa – a kindergarten teaching assistant – broke down in front of one her colleagues, Allison Malouf (40), while explaining the family’s dire situation.
Allison, who teaches the couple's youngest daughter, Mackenzie (9), didn't hesitate in offering a kidney to Neil after her husband had donated a kidney to save a stranger eight years prior.
Unbeknown to the family, another teacher at the school, Britani Atkinson (44), had also registered as a potential donor for Neil in secret – hoping to save the family further disappointment if she was rejected.
“The diagnosis came as a huge shock to us – we were just enjoying our first year of marriage,” Lisa said.
“When I was denied [the chance] to donate a kidney, panic, fear and sheer anger all made me a prisoner of my own mind.
“The only thing I wanted to do in my life was to give a piece of myself to save my husband – but I couldn’t.”
She says she knew that she had only one other option: to find a donor for her Neil.
“I knew that Allison’s husband had donated a kidney eight years [before], and I wanted to tell her about it all because I knew she’d understand the donor process and the emotions that accompany the journey,” Lisa said.
“Her immediate response was, ‘I want to donate my kidney. Let’s get me tested for Neil.’ I was so shocked and politely declined her offer – but she insisted.”
Allison was approved as a donor in May last year, and soon after Britani was also approved.
But because of conflicting blood types and kidney sizes, neither of the pair could directly donate to Neil so they registered with the National Kidney Registry.
Allowing incompatible donor-patient pairs from all over the country to set up four-person “chains”, where donors can swop compatible organs. Britani’s universal blood type allowed her to find a match quickly – and, in turn, by September Neil was undergoing surgery.
Despite her initial designated recipient receiving a life-saving intervention, Allison remained inspired to donate a kidney, and after beginning a chain of her own, four others were saved – including a 14-year-old boy.
Now enjoying Neil’s renewed health, Lisa is hoping her story helps to comfort others desperately in search of a kidney.
Within 24 hours of Neil receiving a kidney, his health drastically improved.
“For 15 years, I had watched a downward trajectory in his [kidney] function. It seemed too good to be true that in just a matter of moments the numbers were climbing.”
“I’d seen him so grey and sullen for so many years, but now the colour had come rushing back to his face. It’s nearly impossible to express the gratitude we feel.”
Britani and Allison don’t see their actions as heroic, instead asking why they wouldn’t donate their kidneys as they each have two and only need one.
“I believe people can learn from Britani and Allison’s courage and selflessness by recognizing that any greatness worth achieving usually happens outside your comfort zone, Lisa said.
No comments:
Post a Comment