From WCVB, ABC Affiliate, Boston
'Revolutionary' drug offers first treatment for devastating kidney disease
For Claudia Iliescu, the trunk of her family tree might as well have the letters PKD carved into the base.
The acronym is shorthand for polycystic kidney disease.
"My grandmother had it," Iliescu said. "And many uncles and aunts and cousins of my mom had it."
She said about half of the relatives on her maternal side have been diagnosed.
"They had persistent, consistent pain," Iliescu said. "And complications."
Iliescu's family has an hereditary form of the disease called autosomal dominant PKD. It's progressive, causing fluid-filled cysts to develop and multiply in both kidneys.
"And as the cysts grow, you feel fullness in your abdomen," she said. "You cannot eat anymore very well because everything's compressed."
The disease has no cure. Instead, as the kidneys fail, patients undergo dialysis or a kidney transplant.
This poor prognosis has devastated Iliescu's family for generations. She remembers thinking that it's her turn now.
"It's always in the back of your head," she said. "You always think, 'OK, so this is what's coming and there's nothing I can do about it.'"
That changed when Dr. Ronald Perrone at Tufts Medical Center suggested she participate in clinical trials for the drug tolvaptan.
The treatment helped Iliescu. Now, the Food and Drug Administration agrees, it can help other patients, too.
"This is the first disease-modifying drug for polycystic kidney disease," Perrone said. "So it's really revolutionary."
Tolvaptan is now available under the brand name Jynarque.
The drug won't reverse the symptoms of PKD, but the trials showed it delays them. It also decreases the rate of kidney pain, stones and infections.
"For patients, it gives them some hope rather than the bleak future that many of them might have envisioned for themselves," Perrone said.
Even with the trial over, Iliescu continues to take tolvaptan. She still has cysts, but they don't appear to be growing.
"Even if I need a transplant or dialysis in my late 60s or 70s, that's a much better outcome than needing it earlier," Iliescu said. "Just slowing down the progression of the disease is fantastic."
PKD Research
From The Conversation

It doesn’t look like a kidney, but this ‘kidney-on-a-chip’ is a breakthrough for new drug testing. Alex Levine, CC BY-ND
Getting a new pharmaceutical from an idea in the chemistry lab to market takes many years and billions of dollars. Each year just several dozen new drugs are approved for use in the United States.
Human “organs-on-chips” are leading a revolution in drug safety testing. These devices use human cells to model the structure and function of human organs and tissues. By testing the potential effects of drugs on different organs faster than traditional methods, organs-on-chips can reduce the need for animal studies and better predict which new drugs will effectively treat human disease.
As part of an interdisciplinary research team, we’re working on a kidney-on-a-chip to improve our understanding of how kidney diseases begin and which drugs can safely treat them.
Human “organs-on-chips” are leading a revolution in drug safety testing. These devices use human cells to model the structure and function of human organs and tissues. By testing the potential effects of drugs on different organs faster than traditional methods, organs-on-chips can reduce the need for animal studies and better predict which new drugs will effectively treat human disease.
As part of an interdisciplinary research team, we’re working on a kidney-on-a-chip to improve our understanding of how kidney diseases begin and which drugs can safely treat them.
Quicker and better testing
Historically, laboratory testing for new drugs is performed in cells grown in dishes or flasks. If a drug passes initial screening tests in vitro, researchers next test it in vivo in live animals to determine the effects of a new drug on a whole system instead of just one cell type at a time. Finally, after many years of laboratory investigation, researchers will test a promising new drug in people to see if it is safe and effective.
The problem is 9 out of 10 of these drugs never make it from small-scale human tests to the patient because they turn out to be ineffective or toxic, even if they showed promising results in early testing.
Organs-on-chips have the potential to completely transform that system. Ranging from the size of a fingernail to that of a credit card, they’re composed of fluid channels and tiny chambers that contain human cell samples. Organs-on-chips in development in labs around the country include kidney, lung, liver, intestine, skin, brain, heart, bone and reproductive systems.
In an organ-on-a-chip, flowing liquid supplies the cells with oxygen and nutrients, similar to the way blood sustains cells in the human body. It’s this constant flow that makes these devices special. Cells grown in organs-on-chips devices act more like cells in a human organ than do cells grown in flat dishes without flow.
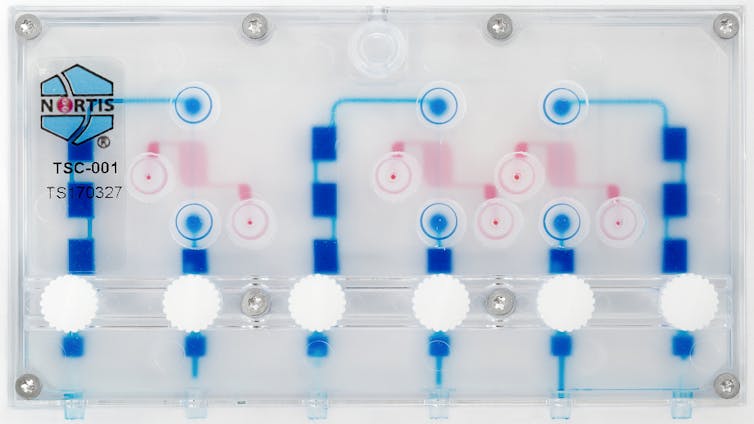
Case of the kidney-on-a-chip
Kidneys are incredibly important to overall human health. The two fist-sized kidneys remove drugs and unwanted compounds from the body and play a critical role in maintaining proper salt and water balance, blood pressure and vitamin D and bone health. Genetic conditions and even commonly administered medications can, in some circumstances, damage the kidneys.
In the U.S., 15 percent of adults have kidney diseases. But most don’t even know it, because kidney diseases often display no symptoms until the condition is very advanced. There’s a pressing need to understand how kidney disease begins, and to develop new safe and effective treatments.
Here at the University of Washington, our kidney-on-a-chip research team is composed of scientists from many different disciplines, including pharmacy, pharmaceutical sciences, nephrology (kidney medicine), toxicology, biochemistry and bioengineering.
In partnership with Nortis, Inc., a local biotechnology company, our team has created a small device — the size of a business card — with up to three tiny tubes, each one-thousandth the size of a drop of water, containing 5,000 human kidney cells. When tiny amounts of fluid are pumped through the tubes, the kidney cells are exposed to important signals that help the cells in the chip behave as if they were in a live kidney.
We’ve found that the kidney cells release signals – called biomarkers – of injury when exposed to known kidney toxins. Our research showed that cells on the chip released markers of injury commonly seen in the urine of people with kidney damage. Testing with the older method, using cells on plates, did not show any damage with the same treatment. This suggests that the kidney-on-a-chip may be better than existing methods at predicting if a new drug will cause kidney damage in humans.
Connecting organs-on-chips to mimic systems
Now that we’ve had these promising results, scientific teams across the country are starting to connect different organs together to replicate a more complex, multi-organ system, to give greater insights into how drugs affect people. For example, we were able to connect a liver-on-a-chip to a kidney-on-a-chip to learn how a plant extract used in some herbal medicines, called aristolochic acid, damages kidney cells. This chip-to-chip investigation reinforces the need for interconnected organs-on-a-chip to replicate the complex mechanics in the human body.
In the coming year, our kidney-on-a-chip project will be one of several sent to the International Space Station where low gravity speeds up changes in cells, sometimes causing health problems for astronauts. The Space Station could be the perfect place to find out more about kidney diseases in weeks, rather than years or decades.
Organs-on-chips can also be used to discover new drug targets. Our team is evaluating the kidney-on-a-chip as a tool to personalize drug selection and dosing in people with kidney cancer, polycystic kidney disease and chronic kidney disease. Other organs-on-chips labs around the country are studying diseases of the immune system, brain, lungs, heart and blood vessels. By working together, dozens of research teams are developing this new technology to revolutionize drug discovery, leading to the development of better and safer medications for all.
From Nasdaq.com, Press release
Bardoxolone Methyl Improved Kidney Function in Patients With Autosomal Dominant Polycystic Kidney Disease and IgA Nephropathy in the Ongoing Phase 2 Phoenix Study
Reata Pharmaceuticals, Inc. (NASDAQ:RETA), a clinical-stage biopharmaceutical company, today announced that positive interim data from the autosomal dominant polycystic kidney disease (ADPKD) and IgA nephropathy cohorts of the ongoing, open-label, Phase 2 PHOENIX trial are being presented by Pablo E. Pergola, M.D., Ph.D., Research Director, Renal Associates, PA, San Antonio, at the European Renal Association and European Dialysis and Transplant Association (ERA-EDTA) meeting in Copenhagen.
The ADPKD cohort of PHOENIX enrolled 31 patients, and available data demonstrate that bardoxolone methyl (bardoxolone) significantly improved kidney function in ADPKD patients as measured by their estimated glomerular filtration rate (eGFR). Bardoxolone-treated patients showed a mean improvement of 6.6 mL/min/1.73 m2 at Week 4 (n=31; p<0.0001), increasing to 12.0 mL/min/1.73 m2 at Week 12 (n=8; p<0.0001) from a mean baseline eGFR of 47.7 mL/min/1.73 m2. The IgA nephropathy cohort enrolled 26 patients, and data were reported through Week 8. Bardoxolone-treated patients showed a mean improvement of 8.4 mL/min/1.73 m2 at Week 8 (n=9; p<0.0001) from a mean baseline eGFR of 46.2 mL/min/1.73 m2. No drug-related serious adverse events have been reported, and reported adverse events have generally been mild to moderate in intensity. Full data for the primary endpoint of change in eGFR at Week 12 for the ADPKD, IgA nephropathy, and type 1 diabetic chronic kidney disease (CKD) cohorts of PHOENIX will be available in the third quarter of 2018.
"Bardoxolone has now produced large improvements in kidney function in a high percentage of patients spanning 11 trials and five different forms of chronic kidney disease," said Dr. Pergola. "These observations suggest that bardoxolone is addressing pathogenic pathways of inflammation and fibrosis that contribute to the loss of kidney function in patients with chronic kidney disease."
Additionally, Dr. Christoph Wanner, M.D., Chief of the Division of Nephrology and Hypertension at the University Hospital of Würzburg, Germany, gave an oral presentation at ERA-EDTA entitled "Bardoxolone Methyl Prevents eGFR Decline in Patients with Chronic Kidney Disease Stage 4 and Type 2 Diabetes ─ Post-hoc Analyses from BEACON." The analysis demonstrated that patients randomized to bardoxolone were more than 50 percent less likely than patients receiving placebo to experience events that predict kidney failure. The authors of the abstract concluded that bardoxolone preserves kidney function and may delay the onset of kidney failure in patients with type 2 diabetes and stage 4 CKD. The abstract was named a Ten Best Abstract by the Paper Selection Committee of ERA-EDTA.
"Bardoxolone's improvements in kidney function in patients with ADPKD and IgA nephropathy are consistent with improvements observed in other forms of CKD, which have been durable and predictive of retained eGFR benefit after withdrawal of drug in prior trials," said Colin Meyer, M.D., Reata's Chief Medical Officer. "Additionally, selection of the BEACON outcomes analysis for oral presentation at one the world's top nephrology meetings, six years after BEACON concluded, reinforces the importance of the study's results. The durable increases in eGFR associated with a 50 percent reduction in outcomes inspire optimism for bardoxolone's potential to delay or prevent dialysis in CKD."
Living with PKD
From Great Reporter
Launch of the ADPKD Patient Routemap: an interactive new resource to help empower patients and families
ERA-EDTA Congress 2018 sees the launch of the ADPKD Patient Route Map, an interactive resource designed to help educate and empower people affected by autosomal dominant polycystic kidney disease (ADPKD).
‘The ADPKD Patient Route Map is a great example of how patients and experts can work together’, says Tess Harris, PKD International President and an ADPKD patient. ‘Ultimately the idea is to help everyone affected by ADPKD cope better with the disease and get all the care, support and information they need, at the right time’.
The ADPKD Patient Route Map is freely available to download from the PKD International website (www.pkdinternational.org/adpkd-route-map).
The Route Map explains the types of care and support that patients and families should expect from their health service. The aim is to help patients and carers to manage their own health with their healthcare team, to talk about ADPKD with their nephrologist, to participate in making decisions about their own care, and to make the best use of available care and support services.
The Route Map was developed jointly by the European ADPKD Forum (EAF). EAF is an international group of experts from the fields of nephrology, genetics, hepatology and advocacy, and PKD International, the international ADPKD patient support group alliance.
The idea for the Route Map came from an EAF Round Table meeting involving patients and representatives from various European-level societies of medical specialists involved in ADPKD care and kidney patient organisations, in January 2016. The resulting ‘EAF Multidisciplinary Position Statement on ADPKD Care ’, recently published in the April edition of Nephrology Dialysis and Transplantation, explains the principles and evidence base for the Route Map.
The Route Map presents in lay terms what ADPKD is, how it is diagnosed, assessed and managed over the course of the disease – including self-care measures that patients can take to stay as healthy as possible. It also covers kidney complications (such as cyst infections and kidney stones), pain management and major non-kidney manifestations (such as liver cysts). It gives advice on issues such as genetics and genetic testing, family planning, and coping with the effects of ADPKD on wellbeing, work and finances. Finally, it outlines opportunities for patients to participate in research, registries and highlights the role of the European Rare Kidney Disease Network (ERKNet).
As Prof. Albert Ong (Sheffield, UK), a co-author of the Route Map put it: ‘We’ve tried to map out ADPKD along the course of a lifetime. What’s great about the Route Map is that it’s not just a book of facts – it’s attractive and interactive, allowing people to look at different topics according to the different stages of their own journey.’
The Route Map allows readers to reveal further information on key topics and messages of advice, experience and support provided by patients and their family members across Europe. Checklists are provided to help patients and families get the most out of consultations, and healthcare teams ensure that patients are always at the centre of their care pathway.
Co-author Dr Vicente Torres (Rochester, MN, USA) said: ‘The Route Map should be very useful to nephrologists – to help us to inform and empower our patients and to ensure that our services are truly patient-centred.’
We are urgently in need of kIdney donors in Kokilaben Hospital India for the sum of
ReplyDelete$500,000,00,WhatsApp +91 8681996093
Email: hospitalcarecenter05@gmail.com
Bardoxolone methyl (also known as “RTA 402” and “CDDO-methyl ester”) is an orally-available first-in-class synthetic triterpenoid belonging to the antioxidant inflammation modulator (AIM) class. It is the most potent known inducer of the Nrf2 pathway to enter clinical development and works to suppress both oxidative stress and inflammation. Bardoxolone methyl
ReplyDelete