PKD Research
From Nephrology News

“Dialysis is a promise unfulfilled,” said Jonathan Himmelfarb, director of the Kidney Research Institute and Joseph W. Eschbach, MD, Endowed Chair in Kidney Research at the University of Washington School of Medicine.
What is the promise? For that answer Himmelfarb, during his session at the National Kidney Foundation Spring Clinical Meetings, referred to quotes from the pioneers of hemodialysis, Willem Kolf and Belding Scribner.
“If we are going to keep patients alive by artificial means, we then incur the responsibility to see that it is a good life and an enjoyable life,” Kolff said in 1968.
“If the treatment of chronic uremia cannot fully rehabilitate the patient, the treatment is inadequate,” Scribner said in 1963.
Dialysis keeps people alive, but it doesn’t restore health, Himmelfarb said. And it doesn’t fully rehabilitate.
To provide a better treatment option for end-stage renal disease patients, you first have to know what a patient wants from treatment, Himmelfarb said. Our current understanding of what patients want is incomplete.
He referred to data from the Standardized Outcomes in Nephrology–Hemodialysis (SONG-HD) Initiative for a glimpse of what is important to hemodialysis patients. According to a survey, the most important outcomes for patients were fatigue, ability to travel, free time away from dialysis, impact on family, and ability to work.
Simply put, ESRD patients want to live their lives as unrestricted as possible.
Being confined to a treatment schedule that hinders their ability to visit out-of-state family and take vacations, or being too washed out and tired to even take a trip, or perform daily tasks, severely diminishes their quality of life. According to the SONG data, and other studies, quality of life is more important to many patients than mortality.
There are also physiological obstacles to improving treatment. Our understanding of uremia is incomplete, Himmelfarb said. And our measure of dialysis adequacy is inadequate.
Developing a wearable artificial kidney
The logical progression of dialysis treatment improvements, according to Himmelfarb, would be portable (think a smaller, lighter version of the NxStage System One), wearable, and implantable.
And through the Kidney Research Institute and the University of Washington, Himmelfarb is working on the wearable.

In 2015, they tested a device developed by Victor Gura, MD, FASN, from the David Geffen School of Medicine at UCLA, on seven hemodialysis patients.
Himmelfarb said they felt good about the trial. They learned a lot, the patients were excited about the freedom the device gave them, and outcomes were good. [Read more]
From Science Daily
Fruit flies used to further our understanding of cysts, cancer
According to PKD International, 12.5 million people are affected by polycystic kidney disease. There is no known cure. But that may one day change, thanks in part to new research by a Concordia biology researcher.
In a study, recently published in PLOS Genetics, Chiara Gamberi and her coauthors developed an innovative fruit fly-based model of the types of harmful cysts that can form on kidneys. The model has enormous potential for assisting the study of how cells proliferate in polycystic kidney disease and cancer.
But what do fruit flies have to do with it?
"The human and fly genomes show a surprising level of similarity. In fact, gene relationships, or genetic pathways, are virtually identical between human beings and fruit flies," explains Gamberi, who is affiliate assistant professor of biology in Concordia's Faculty of Arts and Science.
"Most human organs have fly counterparts. That's a great advantage we can leverage to study the functions of disease-associated genes, and also to identify possible methods of combating those diseases."
Kidneys are particularly challenging to investigate because of the difficulties of isolating the nephrons -- tiny tubes in the kidney that filter substances from body fluids. The fruit fly equivalent, small though it is, acts as an effective stand-in, with the added advantage of allowing researchers to rapidly assess genetic and chemical influences because of the fruit fly's short lifespan.
Gamberi and her coauthors reported the first example of renal cysts in the fruit fly species Drosophila melanogaster.
Through an interdisciplinary approach that included genetic analyses, molecular biology, micro-dissection and drug screening, they have begun deciphering the biochemical pathways through which kidney cysts form. They have also established screening methods to identify drug candidates. The results will help medical professionals identify new treatment targets and methods for certain kidney diseases and cancers.
"Our findings both validate and prompt further use of this first-in-kind fly model of kidney cyst formation in order to pinpoint the molecular and cellular mechanisms at work," says Gamberi.
"I hope that our studies will help define the precise cellular and molecular defects underlying kidney cyst formation," she adds.
"This will also give greater insight into diseases like cancer, in which certain types of cells proliferate. Ultimately, this will help to select targets and drugs for therapeutic interventions aimed at reducing cyst formation and restoring nephron function."
Understanding PKD
From The Lancet, by Albert C M Ong
The term polycystic kidneys was first coined by Felix Lejars in 1888 to describe the clinical signs of bulky kidneys following earlier anatomical and pathological descriptions. Polycystic kidney disease (PKD) is now known to encompass not only the autosomal dominant form (ADPKD) but also includes many rare forms. Since the work of Lejars and his contemporaries, the pathogenesis of PKD has continued to provoke much debate and study.1 [Read more]
Gift of Life
From News & Record, Greensboro, By Tina Firesheets
Lives intertwined for transplant recipient, donor family

Mona O’Bryant and Terri Harris have been friends since 1988.
Harris began working as a clerk at Smith Moore Leatherwood law firm three years after O’Bryant joined the firm.
Their bond strengthened when both became pregnant with their sons in 1996. The working mothers even shared a nanny when their sons were young. O’Bryant’s son, James, and Harris’ son, Mitchell, practically grew up like brothers. They remain friends today.
But the bond between O’Bryant and the Harrises is even stronger now. O’Bryant has developed a lifelong connection to Terri’s husband, George. Literally. She has one of his kidneys.
“They have been family for a long time,” O’Bryant says. “Terri and George have always supported me, and it did not surprise me when George made the offer.”
Harris is mostly recovered from the transplant, which was completed Jan. 10. O’Bryant’s recovery has taken longer, but she has more energy and recently returned to the law firm’s office.
O’Bryant is one of four sisters — all of whom have received a diagnosis of polycystic kidney disease, or PKD. The disease causes cysts to form and grow on the kidneys, eventually impeding kidney function. There’s no cure for it, and the cysts never go away.
Their father, who also had PKD, had a kidney transplant in 1983. He has since died. PKD is an inherited disorder, so their children also have a 50 percent chance of having it. Because of this, most family members couldn’t be considered as kidney donors.
O’Bryant, who learned in the mid-1980s that she had PKD, began talking with her doctors about a kidney transplant in early 2016. They predicted that she could need a transplant in two to five years. But her creatinine levels escalated significantly in the following months.
Creatinine is a chemical waste molecule generated from muscle metabolism. It’s transported through the bloodstream to the kidneys, where it’s disposed of in the urine. High levels indicate poor kidney function. Those levels increased so much that by the end of the summer, O’Bryant began discussing dialysis treatment with her doctor.
Then Harris made his offer.
It can take years to find a match for people needing kidney transplants. Regionally (in the Carolinas, Virginia, Kentucky and Tennessee), 44.4 percent of donors were genetically related to the recipient, according to Betty Crandall, the administrative director of transplant services for Wake Forest Baptist Medical Center in Winston-Salem. Some people in need of transplants spread the word through social media.
Crandall says there are increasing numbers of donors and recipients who have met that way. Each year, a few people also decide to donate a kidney anonymously to anyone on the list, she says.
O’Bryant would rather not draw attention to herself and did not solicit donors, but Terri Harris generated an email campaign among their co-workers and friends.
Her husband jokes that he offered his kidney to O’Bryant because he didn’t think his would be selected.
“I was thinking lots of people would want to be tested (to be a donor),” he says. “I was thinking that I would lead the charge.”
Other people were interested, but the testing process for donors can be extensive. Living donors must be between the ages of 18 and 60 and in good health. They should not have had high blood pressure, diabetes, or kidney or heart disease. They also undergo phone interviews and a series of visits that include blood work, X-rays and meetings with various members of the living-donor team.
“They kept asking if we’d ever had a disagreement,” Harris recalls. “I finally asked, ‘Why do y’all keep asking me that?’ ”
It was to make sure that he wasn’t offering his kidney out of guilt or obligation.
Harris says he doesn’t consider his generosity anything out of the ordinary and even jokes that he did it because “I think (Mona) will put in a good word for me at the Pearly Gates.”
But something else also motivated this father of two:
“I gave Mona the kidney knowing that James needed his mother to be healthy and happy.”
From Buffalo Bulletin, Wyoming, by Jen Sieve-Hicks
Gift of Life
From News & Record, Greensboro, By Tina Firesheets

Mona O’Bryant and Terri Harris have been friends since 1988.
Harris began working as a clerk at Smith Moore Leatherwood law firm three years after O’Bryant joined the firm.
Their bond strengthened when both became pregnant with their sons in 1996. The working mothers even shared a nanny when their sons were young. O’Bryant’s son, James, and Harris’ son, Mitchell, practically grew up like brothers. They remain friends today.
But the bond between O’Bryant and the Harrises is even stronger now. O’Bryant has developed a lifelong connection to Terri’s husband, George. Literally. She has one of his kidneys.
“They have been family for a long time,” O’Bryant says. “Terri and George have always supported me, and it did not surprise me when George made the offer.”
Harris is mostly recovered from the transplant, which was completed Jan. 10. O’Bryant’s recovery has taken longer, but she has more energy and recently returned to the law firm’s office.
O’Bryant is one of four sisters — all of whom have received a diagnosis of polycystic kidney disease, or PKD. The disease causes cysts to form and grow on the kidneys, eventually impeding kidney function. There’s no cure for it, and the cysts never go away.
Their father, who also had PKD, had a kidney transplant in 1983. He has since died. PKD is an inherited disorder, so their children also have a 50 percent chance of having it. Because of this, most family members couldn’t be considered as kidney donors.
O’Bryant, who learned in the mid-1980s that she had PKD, began talking with her doctors about a kidney transplant in early 2016. They predicted that she could need a transplant in two to five years. But her creatinine levels escalated significantly in the following months.
Creatinine is a chemical waste molecule generated from muscle metabolism. It’s transported through the bloodstream to the kidneys, where it’s disposed of in the urine. High levels indicate poor kidney function. Those levels increased so much that by the end of the summer, O’Bryant began discussing dialysis treatment with her doctor.
Then Harris made his offer.
It can take years to find a match for people needing kidney transplants. Regionally (in the Carolinas, Virginia, Kentucky and Tennessee), 44.4 percent of donors were genetically related to the recipient, according to Betty Crandall, the administrative director of transplant services for Wake Forest Baptist Medical Center in Winston-Salem. Some people in need of transplants spread the word through social media.
Crandall says there are increasing numbers of donors and recipients who have met that way. Each year, a few people also decide to donate a kidney anonymously to anyone on the list, she says.
O’Bryant would rather not draw attention to herself and did not solicit donors, but Terri Harris generated an email campaign among their co-workers and friends.
Her husband jokes that he offered his kidney to O’Bryant because he didn’t think his would be selected.
“I was thinking lots of people would want to be tested (to be a donor),” he says. “I was thinking that I would lead the charge.”
Other people were interested, but the testing process for donors can be extensive. Living donors must be between the ages of 18 and 60 and in good health. They should not have had high blood pressure, diabetes, or kidney or heart disease. They also undergo phone interviews and a series of visits that include blood work, X-rays and meetings with various members of the living-donor team.
“They kept asking if we’d ever had a disagreement,” Harris recalls. “I finally asked, ‘Why do y’all keep asking me that?’ ”
It was to make sure that he wasn’t offering his kidney out of guilt or obligation.
Harris says he doesn’t consider his generosity anything out of the ordinary and even jokes that he did it because “I think (Mona) will put in a good word for me at the Pearly Gates.”
But something else also motivated this father of two:
“I gave Mona the kidney knowing that James needed his mother to be healthy and happy.”
PKD Life
Caught in a Catch-22
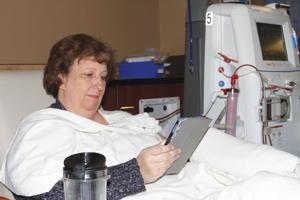
For 25 years, Dawn Crider of Buffalo lived with the knowledge that someday she would require kidney dialysis and maybe a kidney transplant. At age 20 she learned that she had inherited polycystic kidney disease – the same disease that would eventually cause the kidney failure that killed her mother and grandmother.
So last summer, when a routine blood test revealed that Dawn was in end-stage renal failure and had only had 3 percent kidney function, it was a shock, but not one she was unprepared for.
“I knew my all my life that I’d end up on dialysis,” Dawn said. “But it was surprising, because most people with 3 percent kidney function would be very sick and in the hospital, but I felt fine.”
Next came an extensive round of tests and exams, and two days before her 46th birthday, Dawn traveled to the Watt Dialysis Center in Sheridan to begin her first dialysis treatment. She now receives dialysis in Sheridan three times weekly for four hours a session.
“I call it doing my oil change,” she said. “They circulate the blood through a machine and it cleans the blood, and then they pump it back into me.”
She passes the time by reading. She tried to take up knitting to fill the hours, but with one arm tethered to a dialysis machine, knitting didn’t come easy.
Though she could be a candidate for a kidney transplant, she is not currently on the transplant list.
Dawn is caught in a medical insurance Catch-22. As a patient with end-stage renal failure, she automatically qualifies for Medicare. And she also purchased a private, secondary insurance plan to cover expenses not covered by Medicare.
Her dialysis treatments, which run about $200 a session, or $30,000 over the course of a year, are covered by a combination of payments from Medicare and her private plan.
But her secondary insurance doesn’t cover transplants and Medicare only covers 80 percent of transplant expenses. At approximately $150,000 for transplant surgery, Dawn’s out-of-pocket expenses would be $30,000. And then there’s the matter of expensive anti-rejection medication that Dawn would have to take for the rest of her life after transplant.
“When I first started (looking at insurance plans) it was hours and hours of trying to get everything situated,” she said. “There’s a lot of finagling to make sure it’s all covered, to get medications that are covered.”
Right now, she has two options, both of which she considers unattractive.
Moving out of state – Montana has secondary insurance options that cover kidney transplants. Or quit her job as an administrative assistant at the Buffalo Bulletin.
“If I quit, I could get disability and then Medicaid would kick in, and I understand some people have to go that route,” she said. “But I don’t want to quit my job. I like my job, and I can’t imagine not working.”
She is hopeful that any new health insurance bill that is passed would allow health insurance to be sold across state lines.
“I’m going to see how the whole insurance thing in Washington (D.C.) goes,” she said. “Opening up insurance across state lines would be ideal.”
But for now, Dawn waits and watches the dialysis machine pump her blood through a series of tubes and filters and back into her body.
“I’ve learned to rely on God – it’s a lot to take in,” she said. “I just look to God. That’s one thing I wouldn’t trade for anything. Without this, I wouldn’t have such a strong relationship with him.”

For 25 years, Dawn Crider of Buffalo lived with the knowledge that someday she would require kidney dialysis and maybe a kidney transplant. At age 20 she learned that she had inherited polycystic kidney disease – the same disease that would eventually cause the kidney failure that killed her mother and grandmother.
So last summer, when a routine blood test revealed that Dawn was in end-stage renal failure and had only had 3 percent kidney function, it was a shock, but not one she was unprepared for.
“I knew my all my life that I’d end up on dialysis,” Dawn said. “But it was surprising, because most people with 3 percent kidney function would be very sick and in the hospital, but I felt fine.”
Next came an extensive round of tests and exams, and two days before her 46th birthday, Dawn traveled to the Watt Dialysis Center in Sheridan to begin her first dialysis treatment. She now receives dialysis in Sheridan three times weekly for four hours a session.
“I call it doing my oil change,” she said. “They circulate the blood through a machine and it cleans the blood, and then they pump it back into me.”
She passes the time by reading. She tried to take up knitting to fill the hours, but with one arm tethered to a dialysis machine, knitting didn’t come easy.
Though she could be a candidate for a kidney transplant, she is not currently on the transplant list.
Dawn is caught in a medical insurance Catch-22. As a patient with end-stage renal failure, she automatically qualifies for Medicare. And she also purchased a private, secondary insurance plan to cover expenses not covered by Medicare.
Her dialysis treatments, which run about $200 a session, or $30,000 over the course of a year, are covered by a combination of payments from Medicare and her private plan.
But her secondary insurance doesn’t cover transplants and Medicare only covers 80 percent of transplant expenses. At approximately $150,000 for transplant surgery, Dawn’s out-of-pocket expenses would be $30,000. And then there’s the matter of expensive anti-rejection medication that Dawn would have to take for the rest of her life after transplant.
“When I first started (looking at insurance plans) it was hours and hours of trying to get everything situated,” she said. “There’s a lot of finagling to make sure it’s all covered, to get medications that are covered.”
Right now, she has two options, both of which she considers unattractive.
Moving out of state – Montana has secondary insurance options that cover kidney transplants. Or quit her job as an administrative assistant at the Buffalo Bulletin.
“If I quit, I could get disability and then Medicaid would kick in, and I understand some people have to go that route,” she said. “But I don’t want to quit my job. I like my job, and I can’t imagine not working.”
She is hopeful that any new health insurance bill that is passed would allow health insurance to be sold across state lines.
“I’m going to see how the whole insurance thing in Washington (D.C.) goes,” she said. “Opening up insurance across state lines would be ideal.”
But for now, Dawn waits and watches the dialysis machine pump her blood through a series of tubes and filters and back into her body.
“I’ve learned to rely on God – it’s a lot to take in,” she said. “I just look to God. That’s one thing I wouldn’t trade for anything. Without this, I wouldn’t have such a strong relationship with him.”
No comments:
Post a Comment