From Engineering.com, by Michael Molitch-Hou
It was then that Draper was introduced by the dean of the university to my mother, Dr. Susan Hou, then a nephrologist at Tufts University. Along with my family and her local community, Draper launched a campaign to get Dadi to the United States, where she would receive her first kidney transplant.
Kidney transplants and dialysis are the only methods for treating kidney failure. These solutions, however, have proven problematic for numerous reasons, causing researchers to explore new ways of treating kidney failure, including the construction of artificial, biomechanical kidneys or bioprinting new organs from the patient’s own cells.
Such exciting treatments aren’t yet available to kidney patients, but many people are eagerly waiting for science fiction like artificial and bioprinted kidneys to become a reality. To learn about these technologies and how they could play a role in the lives of people like my godmother, Dadi, and my own mother, I spoke to a variety of researchers in the field.
A Kidney Patient in China
Jump back to 1956, in Shanghai. Dadi and her family learned that she had kidney problems when she was just two years old, because there was blood in her urine. Her doctor’s treatment involved a mix of herbal remedies and Western medicine, but the treatment was not successful, and every time she caught a cold, the disease would be exacerbated. The situation did not change until she was about eight years old, when she met a doctor who tried to strengthen her immune system and, therefore, reduce the frequency with which she would have a cold and blood in her urine. The treatment seemed to work for sometime, but, when the Cultural Revolution occurred, her primary doctor was sent to perform custodial work, according to Dadi. Meanwhile, Dadi’s parents, U.S.-educated professors, were sent to labor camps. A 12-year-old living alone in Shanghai, Dadi said that she sought relief from the foul-tasting Chinese herbal medicine she had to take regularly and decided to skip out on the treatment altogether.
After several years, without the proper medication, Dadi’s body became very swollen and she began excreting protein in her urine. By the time the Cultural Revolution was over and her doctor was allowed to return to his practice, there was little he could do to reverse the damage already done to her kidneys. In China at the time, dialysis was not available, while kidney transplants had only been performeda handful of times at an experimental level.
“I was admitted to the hospital and there was a girl in the same ward with me, a little bit older than me—in her twenties,” Dadi said. “And she just died. I saw her die in the bed right next to me because of exactly the same disease I had. I realized that that could be me. I saw a lot of a doctors, even went to different cities to see famous doctors, and they all said the same thing.” Without a transplant or dialysis, the consensus opinion from all of the doctors that she saw—even those considered the most qualified experts in the country—was the same: her condition was fatal.
Thankfully, Dahai was already in the U.S. where such treatments were available. Draper was able to set up a nonprofit organization with which to legally raise funds for Dadi. Japan Airlines agreed to fund half of her airfare to travel to the United States and, over the course of a couple of months, Draper managed to raise the over $40,000 necessary to perform a kidney transplant.
Made up of about one million filtering units called nephrons, the kidney is essential to filtering the waste that passes through the bloodstream, as well as participating in homeostasis in the body. Within a nephron, the glomeruli are an elaborate tuft of blood vessels that keep proteins inside the blood vessels and filter out other material.
The resulting fluid is passed through a structure called the tubule, where specialized cells in its lining reabsorb water and necessary minerals back into the body, while the remaining waste-containing liquid is sent to the bladder to be excreted as urine.
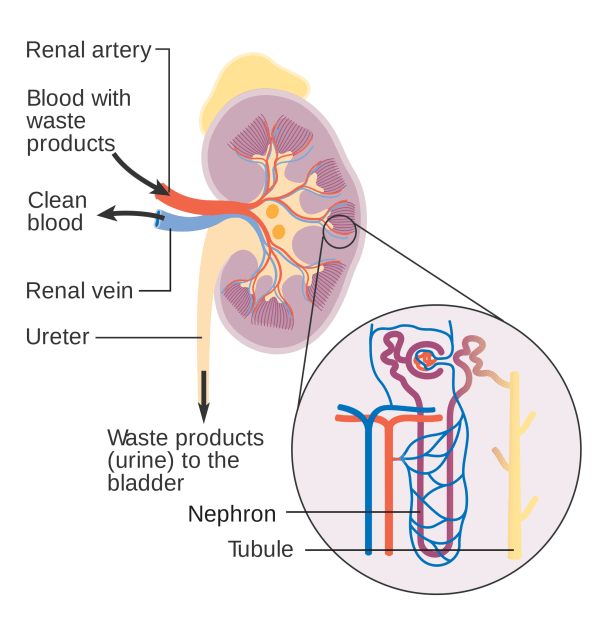
The nephrons don’t just arbitrarily reabsorb what the body needs and get rid of what it doesn’t, but do so at levels appropriate for the body to maintain homeostasis. For instance, if you drink too much water, the kidney will send more of the liquid to be excreted as urine. If you drink too little, the kidney will ensure that more water is reabsorbed into the bloodstream. The same is true for molecules like glucose and calcium. If a disease or toxin disrupts the function of the kidneys, waste may not be disposed of properly or homeostasis may be disrupted.
Kidneys in America
When you think about it, even the most rudimentary forms of organ transplant are fascinating. Taking a vital piece of one person’s body and placing it into another’s is mad science at its best, but getting those organs to work isn’t easy. Not only must the blood types of donors and recipients be compatible, but so must their human leukocyte antigen (HLA) type. HLA antigens are what enable the immune system to distinguish between one person and a foreign body, such as a transplanted organ. Once the immune system recognizes the kidney as being foreign, it treats it like bacterial in an infection and tries to destroy it.
Dahai was set to be Dadi’s donor, but the siblings learned that their crossmatch was positive. Upon introducing her blood to his, their antibodies immediately reacted to destroy the foreign element. This positive crossmatch suggested that Dahai’s kidney would be instantly rejected if it were implanted into Dadi. A transplant from her brother was not the only option, however. “In 1982, a surgeon that received a deceased donor was required to give one kidney to the national waiting list, but was able to give the other to his or her own patient,” my mother explained. “So, when a deceased donor came into Tufts, the surgeon was able to give one of the kidneys to Dadi.”
The donor kidney came from a young person who had died in a motorcycle accident, meaning that the kidney was relatively healthy. Nevertheless, it lasted only a week before Dadi’s body rejected it and the organ had to be removed. “At the time, the immunosuppressants weren’t as effective as what we use today,” Dadi told me. “A new medication called cyclosporine was in experimental trials, but not at Tufts, where I was having my transplant done.”
Dadi was then placed on the national waiting list, as she awaited another deceased donor. This process can take quite a long time; in her case, it was seven years. During that time, Dadi studied to become a nephrology nurse, got married and her life was sustained by peritoneal dialysis (PD).
Peritoneal Dialysis vs. Hemodialysis
Outside of actual kidney transplants, any technology used to treat kidney failure attempts to replicate the function of an actual kidney. This is done through the use of a system that features a semipermeable membrane for removing waste and excess water from the blood.
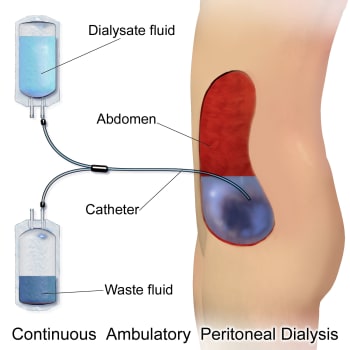
While on PD and studying at nursing school, Dadi said that it was possible for her to fill her abdomen with fresh dialysate before heading to class until, after about four or five hours, she would go somewhere private, drain the waste fluid, and pour in some new dialysate once again. Although this process of continuous ambulatory peritoneal dialysis made it possible for Dadi to go about her daily life while undergoing treatment, she ultimately began to suffer from complications. The glucose in the solution caused her peritoneum to thicken to the point that it no longer functioned as an effective filter.
At this point, she switched to hemodialysis. Instead of an organic membrane within the body, hemodialysis relies on an external machine. Blood flows from a surgically altered blood vessel called a fistula in a patient’s arm into the dialysis machine, which includes a dialyzer made up of hollow synthetic fibers that ultrafilter out waste and excess fluid while dialysate cleans the blood. The cleaned blood is then returned to the body through a second needle. This process continues for three to four hours and must be performed at least three times a week to effectively clean the blood.

By this time, Dadi actually became one of these trained staff members as a registered nurse who oversaw a dialysis unit. As it is for all such dialysis patients, she found it difficult to both work and dialyze three to four hours three days a week. Fortunately for her, she adopted home hemodialysis. This has allowed her to perform the treatment during flexible hours, usually at night while she tries to sleep.
Dadi explained that dialysis is only capable of removing about 15 percent of the waste that must be removed from her body. My mom pointed out that this can be improved by dialyzing longer, more frequently or through improvements in the machine technology. “The efficiency of a dialysis machine is determined by the size and how fast the blood moves through the dialyzer,” my mom explained, “as well as how big the holes are in the dialyzer. They have to be big enough to get rid of the waste, but without removing blood cells and proteins.”
The Artificial Kidney: The Future of Dialysis?
Dr. Shuvo Roy, of the University of California San Francisco, and Dr. William H. Fissell IV, of Vanderbilt University Medical Center, are in the process of developing a unique device that acts almost as a biomechanical, implantable dialysis machine. While it is mechanical in nature, the “artificial kidney” uses kidney cells and silicon membranes to replicate the function of a kidney, providing additional functions beyond current dialysis machines. I asked Dr. Roy to explain exactly how it works.

Key to the device is the use of actual kidney proximal tubule cells, which are grown on silicon nanomembranes, according to Dr. Roy. This makes it possible for water, salts, glucose, amino acids and other small molecules to pass through the device freely. “These nourish the kidney cells, and the porous nature of the membranes also allows the cells to dispose of small wastes, such as carbon dioxide,” Dr. Roy explained. “The silicon nanomembranes also provides immunoisolation for the kidney cells. The immune system relies on fairly large molecules (antibodies) to identify and attach foreign intruders, which are a thousand times larger than small nourishing components such as glucose. These molecules are too large to penetrate the sieve of the membrane supporting the kidney cells.”
Altogether, the device may be more effective than dialysis, not only because it provides continuous blood filtration, but also because it does so with silicon nanomembranes and actual kidney cells. These silicon nanomembranes make it possible to shrink the device down to an implantable size, but also perform better than the plastic components used in existing dialysis machines. Whereas hemofilters for dialysis machines have a surface area of about 2 square meters, the silicon filters that Roy’s lab uses are one-twentieth of the size.
“Traditional dialysis machines remove blood from the patient, filter it through an external machine, and then return the blood to the patient,” Dr. Roy said. “The implanted artificial kidney will allow the filtration to occur continuously, and within the patient’s body, removing the need to be tethered to an external machine. Human kidneys conduct these functions through hundreds of thousands of kidney cells. The artificial kidney performs blood filtration through the use of silicon nanomembranes instead of polymer membranes that are used in conventional dialysis. In addition, the artificial kidney contains kidney cells in the bioreactor to provide biological functions that dialysis simply cannot.”
For the team, the long-term challenges associated with the artificial kidney are keeping it operational and troublefree after implantation. All of the potential issues won’t be known until clinical trials begin in the next year or so. For the time being, Dr. Roy is examining ways for increasing the lifetime of the kidney cells and methods for
ensuring the absence of blood clots. This includes coating the nanomembranes with molecules that make them “blood-friendly.”
As a part of a three-phase program, the lab has already established concept feasibility for externally testing the hemofilter and bioreactor in ICU patients before shrinking the size of the device so that it can be small enough for permanent implantation.
“Currently, we are in Phase II,” Dr. Roy explained. “We are working on engineering refinements to the device components, continuing experiments on the bioreactor to study the conditions that allow the kidney cells to grow and remain healthy, and we have begun a rigorous series of preclinical animal studies for the hemofilter.”
Phase III will begin in late 2017 or in 2018, at which point clinical trials of the hemofilter will begin to demonstrate the device’s safety. This may expedite clinical trials of the combined hemofilter and bioreactor design and, ultimately, pave the way for more streamlined testing of the combined device.
“Once the bioreactor has completed its own set of rigorous preclinical animal studies, we will begin the combined device clinical trials. During clinical trials, we will work with manufacturers to discuss and manage the details of production. Once the clinical trials are complete, we anticipate that the device will be available for patients shortly thereafter,” Dr. Roy said.
Although Dr. Roy’s team uses 3D printing to create plastic housing for the lab’s prototypes and evaluate surgical considerations for the implanted design, no bioprinting is currently used for the kidney cells. That possibility has not yet been ruled out, however. “Bioprinting may be an interesting tool to use in the development of the bioreactor,” Dr. Roy explained. “As bioprinting technology matures for various kidney cells, we could explore it as an advanced tactic to creating a bioartificial kidney.” [Read more]
From Medscape, by Tejas P. Desai, MD
The Monetary Strains of Dialysis Therapy
Think of a nephrologist, and you will invariably think of dialysis. Perhaps no treatment is as synonymous with a kidney doctor as this remarkable procedure. Since its first use in the mid-twentieth century, dialysis has extended and saved innumerable lives while concomitantly helping to usher modern medicine into a new world.
In this world, many of the physiologic functions of the kidney are married with the automation of machines. Elapsing decades have seen refinements in the procedure. Years of research have culminated in an improved understanding of how best to use dialysis to achieve better clinical outcomes.
Today, dialysis machines are smaller, portable, more efficient, and safer for patients. Indeed these refinements have spread throughout the world, and more patients today have access to and receive dialysis therapy than at any time previously.
Despite these advances and the overwhelming, undeniable progress that scientists have made, dialysis therapy remains a challenging option for many patients. Perhaps that is because kidney transplantation (also starting near the mid-twentieth century) has arguably surpassed dialysis progress in two important areas: clinical outcomes and long-term financial costs. Indeed, the latter appears to be the "Achilles heel" for dialysis. The passage of time has not yielded a sufficiently cost-effective model for chronic dialysis administration. Providing modern dialysis therapy to patients is increasingly expensive no matter where in the world you are. The monetary strains are apparent to many global nephrology providers.
Couple the expense with clinical outcomes that have fallen behind those seen after kidney transplantation, and many wonder if it is time to limit the use of dialysis. A number of providers and governmental bodies have linked the outcomes of dialysis with its costs and the costs for alternative care. Many have concluded that "less bang for more bucks" does not justify dialysis administration as it currently exists.
Four Things to Consider in the Ethical Delivery of Dialysis
So can providers develop rational constraints, possibly grounded in the ethics of modern medicine, to simultaneously provide dialysis therapy to patients and ease the financial strain shouldered by those patients (or their national/local governments)? A recent publication in the Lancet tackles the issues surrounding this question.[1]
For those providers who aren't experts in medicine, a good rule of thumb to ensure that our decisions are ethically sound is to question whether we are acting in the best "interest" of our patient. I contend that the real question isn't what's in the best interest of a patient but rather what are the patient's interests.
This article touches on four areas of interest that can affect the ethical delivery of dialysis therapy. To start, the most obvious patient interest is the quality of dialysis care received. Patients and providers want to provide the highest-quality dialysis care, but in many healthcare systems, such care is costly, and those costs are borne by the providers themselves.
The authors note: "Physicians and dialysis centres might also compromise patient care to reduce costs, increase profits, or provide care to more patients." In some cases, the quality of preventive care is strikingly low because existing financial constructs disincentivize providers from investing in such strategies. No single provider can mitigate financial considerations that deter the delivery of the highest quality dialysis care. What we need are institutional or system-level changes to ensure that the patients' interest in quality of care is aligned with financing mechanisms that incentivize its delivery.
A second patient interest focuses on direct medical costs. For many patients around the world, the costs of each dialysis session are borne exclusively by them. Patients in many low-income countries (LICs) or low-middle income countries (LMICs) do not receive subsidized medical care and must pay a la carte for the dialysis therapy they receive. While the actual cost of a year's worth of dialysis is considerably less in LICs or LMICs (eg, US$3200 in India vs US$31,000 in the United States), the vast majority of patients would not be able to afford it. [Read more]
Thanks for posting this interesting blog, nice information keep posting it. Find artificial kidney transplant in hyderabad .
ReplyDeleteVery useful blog. Thanks for sharing this wonderful information, Get here quality dialysis hyderabad .
ReplyDeleteDonate your kidney for the sum of 3 crore Contact Cloud9 k1dney care centre
ReplyDeleteDr Afreen Begam
Call Number: +917899056482
WhatsApp Number : +917899056482
Email: info@cloud9kcarecenter.co.in
Kidney donor needed urgently at Apollo Hospital, we offer huge amount for one kidney only contact me via WhatsApp number: +918122208392 Email: apollohospitalkidneydep@gmail.com
ReplyDeleteKidney donor needed urgently at Apollo Hospital, we offer huge amount for one kidney only contact me via WhatsApp number: +918122208392 Email: apollohospitalkidneydep@gmail.com