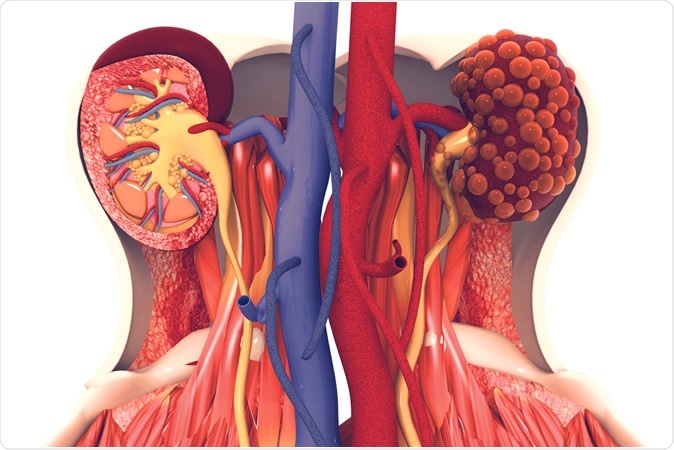
For people with polycystic kidney disease (PKD), life can be a constant cycle of symptoms: aches and pains, abdominal swelling, kidney stones, high blood pressure. At worst, the disease frequently leads to a suite of major issues, including kidney failure, cysts in the liver and vascular problems, including strokes. According to the National Institutes of Health, PKD is a "fairly common genetic disorder," affecting roughly 600,000 people in the United States, with the more common autosomal dominant (AD) form affecting roughly one in 500 to 1,000 people.
"Most patients will eventually form these big cystic kidneys, and they will need dialysis or a
kidney transplant, both of which are not great options," said UC Santa Barbara biochemist Thomas Weimbs, whose research specialty lies in the still somewhat mysterious disease, which has no cure. Meanwhile, treatment of various symptoms and complications put a heavy economic burden on the healthcare system and dramatically lower patients' quality of life.
In a step toward disrupting the cycle that leads to cyst formation in the kidneys, the Weimbs Lab has now uncovered a previously unrecognized mechanism that accelerates cystogenesis. Thought to be a response meant to protect the kidneys, the rapid dilation of the tubules that conduct waste away from the kidneys in the form of urine has been found to be a "third-hit" trigger that results in rapid cyst growth in those with ADPKD.
Their research is published in a paper that appears in the Journal of Clinical Investigation.
The kidneys are the hard-working filtration systems for our blood. Blood enters the nephrons (the kidneys' basic functional unit) where waste and fluid pass through the renal tubules, while cells and proteins stay in the blood. Some fluid and nutrients get reabsorbed into circulation while excess fluid and waste become urine that flows to the bladder. There are about a million such tubules in each human
kidney, Weimbs said.
During this filtration process, waste products—such as
calcium oxalate, calcium phosphate and uric acid—tend to concentrate and precipitate into crystals in the renal tubules. In healthy people, these millions of microscopic crystals form but are flushed away with the urine, while other factors prevent the runaway growth and retention of these crystals in the tubules. The formation and accumulation of these crystals, if left unchecked, could lead to kidney stones.
To prepare to flush out these crystals the renal tubules, it's been found, rapidly dilate, and then return to normal after the crystals have cleared. This dilation is a mechanism that had not been previously recognized, according to Weimbs.
"It was not understood how the bulk of these crystals are flushed out," he said. Until now, stuck crystals were thought to cross through into the kidneys' interstitial tissue to be reabsorbed, he added, but his team's research shows that is not the case for most crystals.
In normal-functioning kidneys, according to the study, the tubule dilation is seen as a protective mechanism. The deposition of oxalate crystals in particular triggers the rapid activation of protein signaling pathways (mTOR and Src/STAT3) that regulate cell growth and proliferation, accompanied by the rapid dilation of the entire tubule system to dislodge the microcrystals.
"In kidneys genetically preconditioned to form these cysts, we found that these crystals can trigger the same dilation, but instead of going back to normal those tubules overshoot and form cysts," Weimbs explained.
In individuals with ADPKD, the rapid and constant tubule dilation is seen as a "third hit" physical injury that results in cyst formation. According to the "third hit" model of cystogenesis, three events must occur to form individual cysts: the first two are genetic mutations, while the third is a physiological damage/repair response, resulting in an overcompensation by the renal tubule that leads to formation of the fluid-filled sacs. Trauma and other assaults to the kidneys are fairly rare, Weimbs said, but the microcrystals could present a persistent and relevant type of injury in ADPKD patients that could trigger the damage/repair response.
The researchers' results suggest that contrary to conventional assumptions that abnormalities in tissue architecture or metabolic abnormalities during ADPKD progression lead to increased kidney stones, the opposite may be the case: More crystals lead to the progression of ADPKD. Additionally, according to the study, it is possible that ADPKD progression and kidney stone formation reinforce each other.
This opens up the possibility that the same well-established practices for keeping kidney stones at bay may also prove effective for slowing the progression of ADPKD. "Our research suggests that the rate of progression could be at least in part determined by something like diet," Weimbs said. Recommendations for preventing
kidney stones, such as avoiding certain foods, increasing water intake and prescription citrate therapy, could also prove beneficial for those with
polycystic kidney disease, he said.
Artificial Kidney
From Daily Bruin, UCLA, BY EMI NAKAHARA
Four-year research collaboration results in potentially lifesaving implantable kidney
An artificial implantable kidney may pave the way for a new treatment for millions of people with chronic kidney disease.
A four-year research collaboration between UCLA and the University of Arkansas created a prototype artificial kidney able to purify human blood and remove its waste products without the need for dialysate, a solution that is typically used for kidney dialysis. Researchers plan to test the technology with a living pig as its first animal model Aug. 29.
The research is funded by the US Kidney Research Corporation, a private research company focused on waterless kidney replacement technology.
According to the
National Kidney Foundation, 37 million people in the U.S. have chronic kidney disease, which is the ninth leading cause of death in the country. It can result in kidney failure, which requires the patient to undergo kidney dialysis – an artificial method of cleaning the blood of toxins and waste – or a kidney transplant to stay alive. Hemodialysis costs Americans $42 billion per year, of which $34 billion is paid via medicare, according to the
Kidney Project at UC San Francisco.
A kidney transplant can replace dialysis as treatment, but there are more than 100,000 people on the waiting list nationwide, said Ira Kurtz, a UCLA professor of medicine who spearheaded the project.
“There’s not enough kidneys to go around,” he said. “Someone who’s waiting for a cadaveric kidney (i.e., a kidney from a deceased person) would have to wait for 10 years in Los Angeles.”
Kurtz, who is also the Kidney Research Corporation’s chief science/medical advisor, said the ultimate goal would be to create an artificial implantable kidney, which does not use water or dialysate fluid. This would not only save on resources and money, but also free up the patients’ time as well, he added.
Currently, the device is roughly the size of a suitcase, but the next goal is to shrink it smaller so it can be easily carried in a backpack, Kurtz said. This way, the patient can carry the device and continuously have their blood be cleaned without needing to go to a clinic or exchange dialysate fluid.
“There are other people who are trying to create a wearable artificial kidney, but those devices still use dialysate solution, so the person has to carry the solution, which we feel is crazy,” he said.
For hemodialysis, patients must be at a clinic or home for three and a half-hour long dialysis sessions three times a week. For peritoneal dialysis, patients undergo the session overnight daily. Hemodialysis requires very large amounts of water and dialysate in order to clean and get rid of toxins and waste from the blood, Kurtz said
“We couldn’t have that infrastructure in an ideal implantable kidney. I think there’s enough water used in hemodialysis per year in the United States to feed all the livestock in the states (for approximately a day and a half),” Kurtz said. “It’s a huge amount of water.”
The new prototype, however, uses filtration and electron deionization instead of dialysate fluid to clean the blood and create urine, Kurtz said. First, an ultrafiltration module takes in blood from the body, while preventing cells and important proteins from the blood from getting into the rest of the device.
Next, electron deionization units, or EDI, transport important ions, such as potassium and sodium, in and out of the blood in order to maintain a crucial balance in their concentration in the body. For example, too much potassium in the blood can lead to a heart attack, Kurtz said.
The device also uses a nanofilter to remove urea, a metabolic waste product, from the blood, and another to prevent important sugars from leaking from the blood into the urine, which can be fatal for the patient, he added.
“Urine has a certain chemistry from cells in the kidney, and we had to simulate them also,” Kurtz said.
Jamie Hestekin, a professor in chemical engineering at the University of Arkansas who led the development of the device, said a challenging part in building the EDI was the partial separation of the various important ions of the blood.
“You don’t want to remove everything,” Hestekin said. “Separating two things from each other completely is possible, but doing partial separation, removing things not needed and keeping those that are needed, is more complicated.”
Minhtri Nguyen, a UCLA professor of medicine specializing in kidney research, said an artificial implantable kidney device that doesn’t require dialysate would be a major advance in the field.
“In theory, it sounds good. But only by testing with an animal model and later in humans we can know for sure it’s effective,” he said. “If it is, it could change the way we practice nephrology.”
However, Nguyen said he was unsure of one aspect of the technology. Although the nanofiltration modules are responsible for removing excess urea from the blood, filtration alone cannot decrease the overall blood urea concentration, he said. Urea cannot be removed by the EDI units because it is not a charged molecule, and also cannot be easily removed by a selective membrane.
“Urea is tricky to remove, but it’s a very major solute,” he said. “If they can’t lower the concentration, that’s a big problem, and they might need to go back to the drawing board.”
So far, the device has performed well in laboratory simulations, using solutions and blood samples, Hestekin said.
He said he is cautiously optimistic for upcoming tests of the device with a live pig.
“It’s been performing really well, with tremendous progress in four years, despite its extremely challenging application,” Hestekin said. “We’re really hoping that the trial can show that with some more incremental progress we can actually get something that works long term.”
From New York Post, by Hannah Sparks
US throws away thousands of kidneys despite donor shortage: study
Despite the 93,000 patients in the US
seeking a kidney transplant, a new report reveals that some 3,500 donated kidneys go unused and even thrown away every year.
A study
published in JAMA Internal Medicine this week found that 17% of donated kidneys in the US were discarded during a 10-year period. By comparison, in France, only about 9% of donated kidneys went unused during the study period.
Why the waste? According to the report, doctors here in the US are less inclined to risk using lower-quality kidneys, even though previous studies have shown that even less desirable kidneys, such as older kidneys and those with abnormalities, are still better than dialysis.
In France, doctors are more willing to use kidneys from deceased patients who suffered from illnesses such as diabetes or hypertension.
Cost may also be a factor in US doctors’ reticence to use affected kidneys, since these transplants may also result in extended hospital stays.
And, without a consistent set of guidelines in the US, some regions discard more kidneys than others based on their own opinions about whether a kidney is viable, according to a 2016 study
by the National Kidney Foundation. That study found that as many as 50% of discarded kidneys could have been transplanted.
The United Network for Organ Sharing (UNOS) — the US’ transplant governing body — attempted to improve the discard rate by creating a kidney donor profile index in 2012, which rates how long it predicts a kidney will hold up. The network even lowered its standards in 2014 so that lesser kidneys would be considered.
The problem is wide-reaching: More than 37 million Americans
suffer from chronic kidney disease, with roughly 5,000 of these patients dying each year on the transplant waiting list.
Still, the number of wasted kidneys continues to rise, up to 30% in some regions,
according to a study published in January 2019.
In the end, “risk-averse” policies in US transplant programs hit patients hardest, write Dr. Ryoichi Maenosono and Dr. Stefan G. Tullius of Brigham and Women’s Hospital, in a commentary article attached to the JAMA study.
“Hospital administrators and patients alike are attracted by superficial five-star ranking approaches that are easy to read but not necessarily reflective of the approach of individual programs aiming to provide their patients on waiting lists with the best opportunities.”
Walk for PKD
From KENS-TV, NBC Affiliate, San Antonio, TX
Seeking a cure with Walk for PKD | KENS Cares
One hundred percent of each donation funds lifesaving research to find treatments and a cure.
The San Antonio Walk for PKD is your chance to take a small step and make a big difference in the lives of those who have polycystic kidney disease.
One hundred percent of each donation funds lifesaving research to find treatments and a cure. Sign up and walk with KENS 5's Sarah Forgany and Cristina Blackwell on Saturday, September 14, 2019.
SATURDAY, SEPTEMBER 14
Register at:
walkforpkd.org/sanantonioCheck-in/Onsite Registration: 7:30 a.m.
Penny Kids Dash: 8:15 a.m.
Walk Begins: 9:15 a.m.
Walk Distance: 1 mile or 3 mile route
OP Schnabel Park, Graff Pavilion
9606 Bandera Road
San Antonio, TX 78250
ABOUT PKD FOUNDATION
The PKD Foundation helps patients and loved ones learn about PKD and how to manage the disease while maintaining a high quality of life. They do this through promoting research, education, advocacy, support and awareness on a national level, along with direct services to local communities across the country.
The PKD Foundation is the only organization in the U.S. solely dedicated to finding treatments and a cure for polycystic kidney disease (PKD) and to improve the lives of those it affects. Since 1982, they have proudly funded $44 million in PKD research and leveraged $1.5 billion in government funding, while serving local communities across the country. Their vision #endPKD.